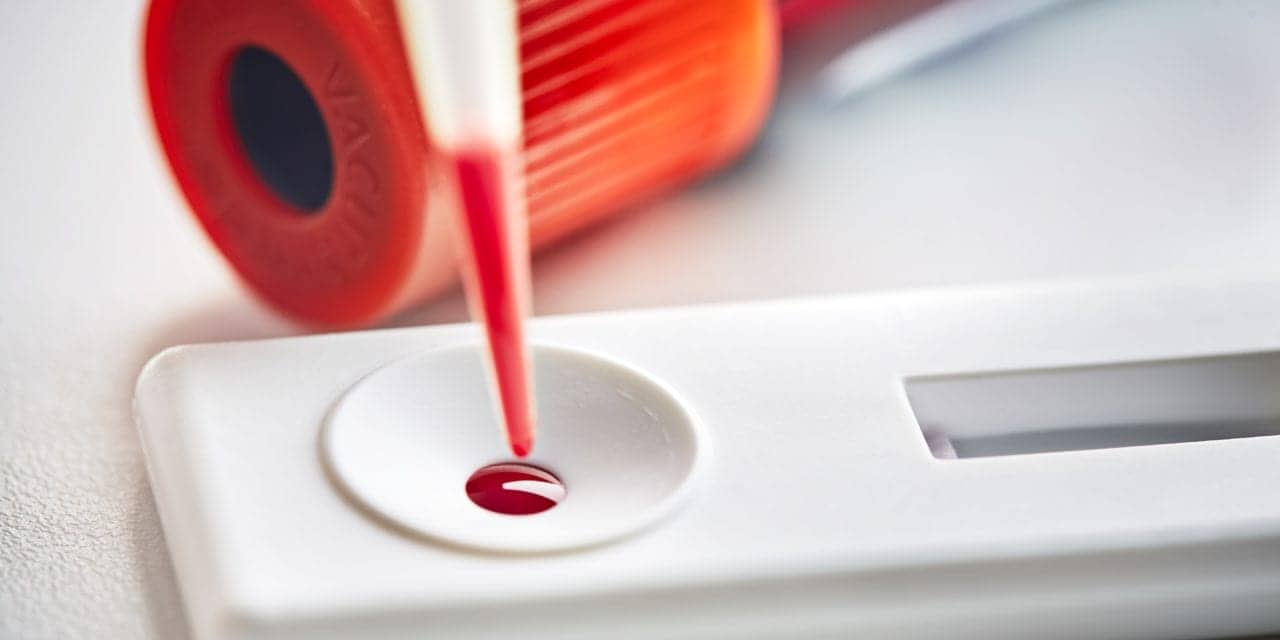
Mologic Ltd, Thurleigh, UK, a developer of lateral-flow and rapid diagnostic technologies, products, and services has completed the development of a next-generation ultrasensitive lateral-flow-based diagnostic technology.
Novel diagnostics based on the technology can be visually read and demonstrate up to a 1,000-fold improvement in sensitivity over current technology. The company has initially demonstrated the technology through the development of low-cost, high-performance test devices for human immunodeficiency virus (HIV) and malaria.
The innovations at the core of the platform can be replicated for any protein or small molecule target, with associated performance gains. The technology represents a major advance in the accuracy and application of commercial lateral-flow assays for rapid diagnostic tests, and supports global efforts to eliminate high-burden epidemics.
To deliver its planned innovations in lateral-flow technology, Mologic established its own center for advanced rapid diagnostics (CARD), with funding and support from the Bill and Melinda Gates Foundation. In the first phase of its project, the CARD team attained the ambitious milestone of 1pg/mL sensitivity in a visually read lateral-flow device. The achievement advances point-of-care testing technology to levels of performance previously attained only with expensive laboratory equipment.
Ultrasensitive rapid diagnostics play an important role in halting epidemics of infectious diseases such as malaria, especially by identifying asymptomatic individuals who nevertheless carry and transmit the infectious organism. CARD’s innovation will facilitate surveillance and earlier disease detection, enabling quicker and more-effective intervention.
“Significantly improving the sensitivity of rapid diagnostic tests has the potential to revolutionize how we care for patients at the point of need,” says Joseph Fitchett, PhD, chief medical officer and head of global health at Mologic. “CARD has delivered high-performance, high-quality, and low-cost innovations that will bring about a step change. It allows easier and quicker access to more effective treatment, which is fundamental in epidemics and neglected disease eradication programs.”
With a new grant of $4.9 million from the Gates Foundation, the second phase of the CARD program is expected to push the boundaries of sensitivity even further, to broaden application of the technology to neglected diseases, and to accelerate product delivery.
In partnership with the Institut Pasteur de Dakar, CARD is also helping to catalyze the DiaTropix initiative and its formation of a responsive diagnostics manufacturing and education facility in Senegal. A key goal of the initiative is to ensure that novel diagnostics for infectious and neglected diseases achieve the target pricing and product profile necessary to support successful deployment at the point of need.
“As we enter the second phase of the CARD program, we are excited to work with Amadou Sall’s team at the Institute Pasteur de Dakar, to ensure successful deployment at the point of need and help eliminate epidemics and control neglected diseases,” says Fitchett.
During 2019, Mologic’s services division expects to open the new technology platform for use by outside partners. Access to the CARD technology will help users to develop new test applications in fields that can benefit from ultrasensitivity at the point of care, such as oncology and agriculture.
“We have exceeded expectations in the delivery of CARD innovations with 1pg/mL accuracy, marking a major scientific advancement in lateral-flow technology,” says Paul Davis, PhD, Mologic cofounder and chief scientific officer. “The parameters for lateral-flow products are now redefined, and we will continue to advance the technology concept to push its limits and applications even further across the world.”
For further information, visit Mologic.
Featured image: Mologic’s visually read lateral-flow device.