Summary:
Vail Scientific, in partnership with Minnetronix, has unveiled a newly designed VSNO sepsis screening device that enhances usability, speeds up diagnosis, and eliminates the need for a blood draw, aiming to improve early detection and patient outcomes.
Key Takeaways:
- Faster Detection: VSNO reduces sepsis test results time from approximately eight hours to under two minutes.
- Non-Invasive Testing: Unlike traditional methods, the device only requires a patient to blow into a breath analyzer.
- Upcoming Clinical Trials: A VSNO verification study is planned for 2025, followed by a final clinical trial and FDA clearance application.
Vail Scientific, which empowers healthcare providers with cutting-edge solutions for early sepsis screening, has unveiled the significant new design of its proprietary VSNO sepsis screening device, which is used for speeding sepsis testing and results for enhanced patient outcomes. Sepsis is a life-threatening complication from infections that damages and causes the body’s organs to fail, especially when it is not detected and treated early. It is the leading cause of death in hospitals and overall cost of hospitalization at an estimated $62 billion. One in three patients who die in a hospital have sepsis.
Sepsis Screening Device Has Successful Proof of Concept
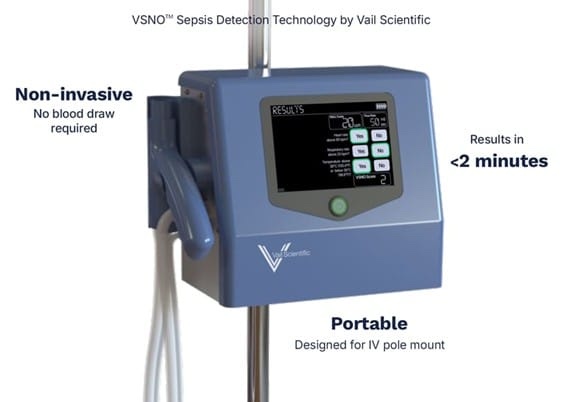
Vail partnered with Minnetronix on the device design after successful proof of concept and feasibility trials for its core technology, which measures sepsis down to single parts per billion. Minnetronix streamlined the device to increase its useability and portability. After a 75% reduction in size, the VSNO can now be mounted on an IV pole. It features a new easy-to-use handle that a patient holds to blow into a breath analyzer that measures nitric oxide levels and, in the VSNO system, serves as a screening tool in the detection of sepsis.
Minnetronix’s team provided Vail and its investors with a reliable project timeline and unit cost for the manufactured device, to support the product launch and commercialization planning. To date, Minnetronix has manufactured a limited number of newly designed devices for a VSNO verification study planned for 2025, which will be followed by a final definitive clinical trial and FDA clearance application.
“We applied a Design-For-Manufacturing mindset to determine how VSNO can be most efficiently produced and scaled to meet the expected market demand,” says Kera West, engineering director at Minnetronix Medical. “Sepsis hasn’t seen innovation in more than a decade. This is such purpose-driven work, and we can’t wait for the new standard-setting sepsis testing process to be in the market.”
VSNO Does Not Require Blood Draw
Unlike standard sepsis tests, VSNO does not require a blood draw and is planned for use at the triage stage, including before a patient enters the hospital. It streamlines the testing process and reduces time to results from approximately eight hours (the time the patient arrives at the hospital to results) to less than two minutes after a patient blows into the device.
“With sepsis, speed and accuracy can help save lives,” says Tom Burke, CEO of Vail Scientific. “The Minnetronix team helped make our technology viable in a device designed for use at the triage stage of a patient workflow. They understood the science and brought their integrated electronics, hardware, and software expertise to the challenge of making VSNO into a better way to address the global issue of sepsis.”
Every hour before treatment, a patient’s chance of dying from sepsis increases by 8%. GlobalData epidemiologists using Sepsis-3 criteria recently forecasted that diagnosed incident cases of sepsis in the eight major global markets will grow from approximately 7.8 million cases in 2024 to 9.5 million cases in 2033. Sepsis is the leading cause of deaths inside hospitals yet as many as 80% of sepsis deaths can be avoided with rapid diagnosis and treatment.
Featured Image: Vail Scientific unveiled the redesign of its VSNO, sepsis screening device, which is 75% smaller than the prior iteration. The device does not require a blood draw in order to make a diagnosis. Photo: Vail Scientific