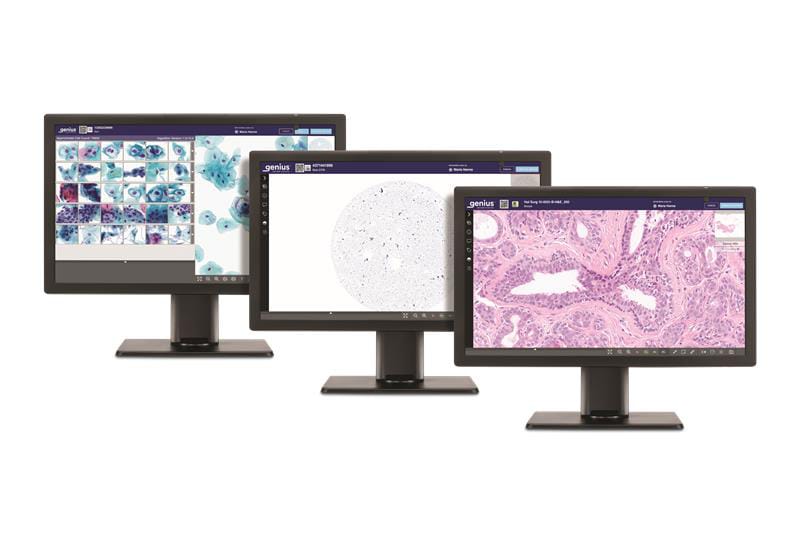
The Genius Digital Diagnostics System is now approved for both cell and tissue specimen imaging in the European Union.
Hologic’s Genius Digital Diagnostics System has received expanded CE marking in the European Union, enabling the platform to image and review both cell and tissue specimens. The system was previously CE marked specifically for cell analysis applications, including cervical cancer screening.
The expanded approval allows the system to perform whole slide imaging, which captures entire slides for review of a broader range of patient sample types. This capability enables European laboratories to consolidate digital workflows using a single platform while supporting pathologists in diagnosing various cancers and other diseases.
With whole slide imaging, the same system can identify pre-cancerous lesions and cervical cancer cells during screenings while also enabling pathologists to review cervical tissue biopsies for diagnostic confirmation. For breast health applications, the technology allows labs to digitize and review tissue from breast biopsies when abnormalities are detected during mammograms.
Addressing Laboratory Inefficiencies
Most laboratories currently rely on multiple systems for reviewing different patient sample types, which can create operational inefficiencies, increase costs, extend turnaround times, and add workload for laboratory staff.
The Genius Digital Diagnostics System uses volumetric imaging technology to capture high-quality digital images of cell and tissue specimens, which are then stored, distributed, and reviewed on a single platform.
“Placing digital pathology at the center of diagnostic workflows has the potential to transform the way we approach cancer diagnosis and prevention,” says Paul van Diest, professor in the department of pathology at University Medical Center Utrecht, in a release. “The ability to image and review more specimen types on a single system will help pathologists think beyond traditional boundaries and bring greater accuracy and efficiency to our work.”
Technical Capabilities and Workflow
The system converts glass slides containing patient specimens into high-resolution digital images using volumetric imaging technology. This process simultaneously captures 14 layers of the patient specimen and converts them into a single, two-dimensional view.
Once processing is complete, entire cases are sent to the system’s image management server for secure storage and automated case management. Cases can then be reviewed locally or remotely to support interpretation and diagnosis.
The expanded CE marking includes additional software capabilities such as remote support, laboratory information system readiness, and new review tools.
“Access to innovative technology in the laboratory can be a foundation for better patient care,” says Jennifer Schneiders, PhD, president of diagnostic solutions at Hologic, in a release. “Expanding our CE marking with digital pathology will help bring advanced technology to more laboratories across Europe and signals another incredible step in Hologic’s innovation pipeline focused on providing accurate and efficient results to support disease screening and diagnosis.”
Hologic’s digital pathology solutions received CE marking in the European Union under the In Vitro Diagnostic Regulation, which is recognized by other countries worldwide. The company will announce commercialization plans by country. Whole slide imaging is not currently available in the US.
Photo caption: Genius Digital Diagnostics System
Photo credit: Hologic