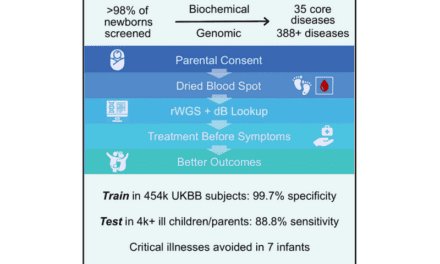
BillionToOne, a next-generation molecular diagnostics company, announced the launch of its first oncology liquid biopsy products, Northstar Select and Northstar Response. The products are currently available for research use with select academic cancer centers.
Northstar Select is a comprehensive pan-cancer somatic mutation profiling panel. Leveraging BillionToOne’s proprietary molecular counting technology, or Quantitative Counting Templates (QCTs), the panel achieves a notable limit of detection for identification of actionable alterations.
Meanwhile, Northstar Response is a methylation-based, tissue-agnostic treatment response monitoring assay. Using molecular counting technology, the assay provides absolute quantification of methylated circulating tumor DNA (ctDNA) burden. The purpose of the assay, when it becomes clinically available in Q1 2023, will be to enable clinicians to monitor patients’ responses to therapies, including in late-stage cancers for which minimal residual disease (MRD) assays may not be as informative or effective.
At this year’s American Association for Cancer Research (AACR) Conference in April, BillionToOne presented data on its methylation assay showing it can distinguish minute changes in cell-free tumor DNA burden as small as 0.5% vs. 0.55% tumor burden[1].
“We are extremely excited to launch our first liquid biopsy products for research,” says Oguzhan Atay, PhD, co-founder and CEO of BillionToOne. “Similar to our category-altering cell-free DNA-based prenatal test, UNITY Screen, our liquid biopsy products aim to achieve a level of performance and differentiation unmatched in the field. In particular, our QCT technology provides precise tumor burden quantification without needing the tumor tissue. We believe that blood-based treatment response monitoring will revolutionize the way cancers are treated and oncology drugs are developed, and our QCT technology helps position us to be at the forefront of this change. By combining Northstar Select and Northstar Response, we aim to be the one-stop-solution for providers treating any late stage solid tumor cancer patient.”
Having completed its internal analysis of the products on six separate cancer types, BillionToOne has released its research program with academic centers to further substantiate the evidence for these two products.
“Scan-based treatment response classification is the gold standard but requires large changes in tumor burden and can be subjective. Results can further be complicated by confounding factors such as pseudoprogression,” says Gary Palmer, MD, medical director, Oncology, at BillionToOne.” Current literature suggests that the ctDNA burden is a highly accurate biomarker for tumor burden and may be an earlier indicator of a treatment response[2],[3]. The application of BillionToOne’s molecular counting technology into cancer care may provide actionable information that saves unwanted toxicity from ineffective treatments, reduce costs, and substantially improves clinical outcomes.”
BillionToOne also announced that it has entered a research collaboration partnership with University of California, San Diego (UCSD). Northstar Select and Northstar Response will be evaluated using late-stage non-small cell lung cancer (NSCLC) patient samples.
“I believe BillionToOne’s technology has offered an opportunity to provide highly accurate measurements for variant allele fractions through the low coefficient of variation it obtains in ctDNA measurements. This is of tremendous importance in the clinic for therapy selection and for response monitoring for NSCLC patients, as tissue biopsy can be difficult to obtain serially. I look forward to the first readout of the study in the next few months,” says Hatim Husain, MD, Medical Oncologist and Associate Professor of Medicine at UCSD, and the Principal Investigator of the study.
BillionToOne is conducting additional clinical studies with other academic cancer centers on different cancer types and is expected to launch the commercial versions of Northstar Select and Northstar Response for clinical use in Q1 2023.
Sources:
[1] Ye et al., “Novel methylation-based, tissue-free ctDNA assay accurately quantifies longitudinal tumor burden changes for precision treatment monitoring”, AACR 2022 Poster #526
[2] Nabet BY, Esfahani MS, Moding EJ, et al. Noninvasive Early Identification of Therapeutic Benefit from Immune Checkpoint Inhibition. Cell. 2020;183(2):363-376.e13
[3] Bratman, S.V., Yang, S.Y.C., Iafolla, M.A.J. et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer 1, 873–881 (2020)