Roche, Basel, Switzerland, has received FDA premarket notification (510(k)) clearance for its CinTec histology test. The clinically validated p16 biomarker test, when used in conjunction with hematoxylin and eosin (H&E) staining, helps pathologists determine which women should receive treatment for cervical precancer.
The test is a part of the Roche cervical cancer portfolio, which includes the Cobas human papillomavirus (HPV) test and the CinTec Plus cytology test.
“The CinTec histology test will help physicians make informed decisions as to the best course of care for patients with high-grade precancerous cervical disease,” says Roland Diggelmann, CEO of Roche Diagnostics. “By improving the consistency of diagnosis across pathologists, it can help ensure the right patients are receiving the best possible treatment for this highly preventable disease.”
Women positive for HPV are at greater risk for having or developing precancerous cervical lesions. Cervical cancer screening can help physicians find and treat these precancerous lesions before they develop into invasive cancers. The CinTec histology test plays a key role when a cervical tissue biopsy is taken as a result of an abnormal cervical cancer screening result, as it provides conclusive visual confirmation of the presence or absence of precancerous lesions. These lesions, if untreated, could eventually lead to cervical cancer.

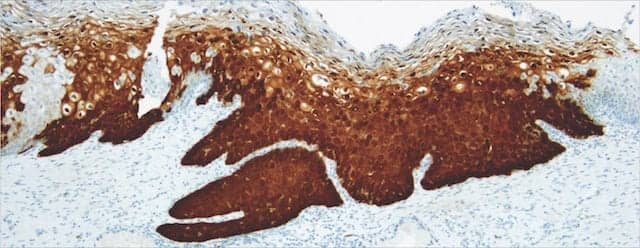
The CinTec histology-stained cervical biopsy specimen demonstrating diffuse, positive status at 10x magnification.
FDA clearance was based on results generated in the cervical tissue adjunctive analysis (CERTAIN) study. Additionally, the use of p16 immunohistochemistry is recommended by the American Society for Colposcopy and Cervical Pathology, the College of American Pathologists, and the World Health Organization, to improve the detection of precancerous cervical disease.
The CinTec histology test uses the p16 biomarker for a more conclusive diagnosis to provide distinctive visual confirmation of precancerous cervical lesions that may be missed by H&E interpretation alone. It is fully automated on the Ventana BenchMark in situ hybridization and immunohistochemistry instruments.
For more information, visit Roche.