This is a sidebar to the CLP June feature, “Personalized Medicine Progresses.”
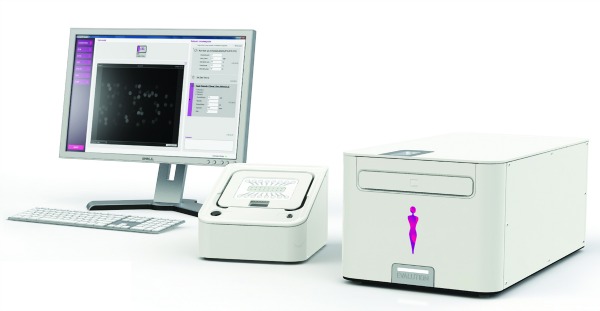
According to Paul Ladestein, managing director of the Evaluation business unit of Biocartis, Evaluation is a very powerful multiplexing detection system that allows laboratories to perform both protein and nucleic-acid biomarker analysis from the same patient in the same assay plate.
This can be used to diagnose disease, to measure pharmacogenetic responses to a therapeutic drug, and also to evaluate the process of drug development and accelerate the translation of novel biomarkers to the clinics. The ability to analyze DNA, methylated DNA, RNA, mRNA, and miRNA at the same time using the same protocol provides clinicians with accurate information for the diagnosis and prognosis of certain diseases (eg, cancer, Alzheimer’s disease, and heart failure).
“The versatility of the system is a clear advantage for the clinical laboratories to consolidate multiple platforms into one single system,” Ladestein says.
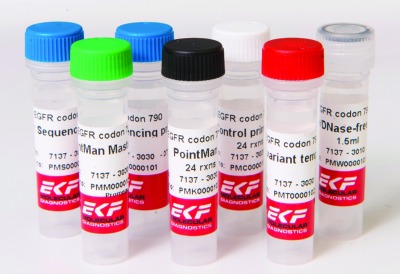
Other diagnostics companies with an interest in personalized medicine have also been active. Global in vitro diagnostics company EKF Diagnostics recently announced that its PointMan DNA enrichment technology for potential use in future cancer testing and treatment was instrumental in the first successful results of collaboration between EKF Molecular Diagnostics and the Institute of Life Sciences at Swansea University, Wales. The researchers demonstrated the detection of gene mutations in blood from samples archived in the Wales Cancer Bank. The PointMan technology was used to analyze the whole blood of cancer patients diagnosed with metastatic melanoma, enabling the identification of gene mutations associated with response to drug treatment.
Crucially, the results observed for mutations in the BRAF gene were consistent with the formalin fixed paraffin embedded (FFPE) tissue samples, FFPE being the laboratory standard method to prepare all biopsy samples for pathology review in order to diagnose the cancer. These results have been confirmed by DNA sequencing, which had failed to identify the mutations prior to sample enrichment through EKF’s PointMan technology.
In addition, EKF Diagnostics acquired Selah Genomics Inc and DiaSpect Medical AB. Sweden-based DiaSpect manufactures desktop hemoglobin analyzers, and Selah, of South Carolina, is a service provider that supplies molecular diagnostics tests for the development of personalized medicine. The integration of DiaSpect’s product line into the existing portfolio of EKF hemoglobin analyzers will enable the provision of an analyzer to meet any need. The patented DiaSpect Hb-system provides instant results from one drop of whole blood. It is suitable for use in dry, humid conditions, and the broad-spectrum photometer together with the reagent-free cuvette offers outstanding reliability and cost-effectiveness. This desktop device will join the company’s point-of-care Hemo Control (Hemo Point H2 in the US) and the handheld, portable Stat-Site hemoglobin analyzer.