More than just Lyme, a number of diseases spread by ticks are on the rise
By Nicole Colby
Lyme disease is by far the most commonly reported tick-borne illness in the United States,1 but it is only one of many known diseases transmitted by these tiny arthropods. And these illnesses are becoming more common. The number of reported cases for all tick-borne diseases has increased by approximately 50% from 2008 to 2018,1 and the incidence of all the reportable tick-borne diseases, with the exception of Lyme, has more than doubled in the 10-year period.2,3
But new or lesser known pathogens are also on the rise. Since 2004, an additional seven bacteria or viruses transmitted by ticks have been identified that can cause illness in humans.3 The effects of tick-borne diseases vary. Some individuals may have only short-term effects that resolve with or without treatment, while others may experience life-long symptoms of their battle with the disease. And some cases are fatal. In addition to Lyme and other more common tick-borne diseases, here are five more obscure and emerging diseases that can be transmitted by ticks.
Emerging from the Heartland
In 2009, two patients in Missouri were admitted to a hospital demonstrating fever, leukopenia, and thrombocytopenia. Both patients reported recent tick bites, which were assumed to be the cause of their illness. Based on the symptoms, infection from Ehrlichia chaffeensis was suspected, but all the tests were negative. Subsequent tests performed on white blood cells from the individuals demonstrated the illnesses were caused by a new virus, eventually named the Heartland virus.4
The Heartland virus was found to be an RNA virus in the Phlebovirus genus, most closely related to the severe fever with thrombocytopenia syndrome (SFTS) virus seen in Asia. Studies supported by the Centers for Disease Control and Prevention (CDC) demonstrated the virus was most likely transmitted by the lone star tick (Ambylomma americanum).5 Since the first cases of this viral illness were identified in 2009, more than 50 additional cases have been reported in the Midwest and southern United States.6
The symptoms of the disease are similar to ehrlichiosis, including fever, fatigue, anorexia, nausea, and diarrhea. Common laboratory findings include leukopenia, thrombocytopenia, and mild to moderate elevation of liver transaminases. A majority of patients infected with the Heartland virus have required hospitalization, but most do recover fully. It is recommended to consider Heartland virus infection for patients suspected to have ehrlichiosis but either have tested negative or do not respond to treatment with doxycycline, the treatment of choice for ehrlichiosis.6
There are no routine tests available to detect this virus or its antibodies. The CDC recommends contacting your state health department for potential testing of patients suspected of infection with the Heartland virus.6
The Powassan Virus
The Powassan virus was identified in 1958 after a young boy in Powassan, Ontario, died of severe encephalitis. Up until 1998, there had been a total of 27 reported cases of Powassan infection in the United States and Canada.7 From 2004 through 2015, the reported case number in the United States alone significantly increased to 70 cases, and it increased again in 2016 through 2019 to 112 cases. These infections were primarily reported from states in the Northeast and Great Lakes region.8
The Powassan virus (POWV) is a flavivirus, as are other vector-borne pathogens including West Nile virus and Zika virus. The closest relative of POWV is the tick-borne encephalitis virus (TBEV), which is highly prevalent in Europe and Asia and infects the central nervous system. In 1997, a second virus similar to POWV was identified in New England that also causes encephalitis; because the new virus was isolated from the deer tick, it was named deer tick virus (DTV).7
POWV and DTV are so closely related they require specific testing to identify them, and so testing for human disease in clinical practice typically does not differentiate which virus caused the infection. But studies suggest that some human illnesses diagnosed as POWV encephalitis could be attributed instead to DTV. Distinguishing between the two causative agents can be important for epidemiological purposes.9
Infection with the Powassan virus may not cause symptoms in all individuals. Some patients could have only minor symptoms, such as fever, headache, vomiting, and weakness, but the virus does have the potential to cause severe disease including encephalitis and meningitis. Of those patients who do develop severe disease, approximately 10% have fatal infections, and about half of those
patients who survive experience long-term effects such as headaches and memory problems.10
Molecular tests are available to detect POWV/DTV RNA in serum, cerebrospinal fluid, and tissue specimens. These tests can demonstrate positive results in the early stages of infection, but sensitivity may diminish as the patient progresses to severe illness. For this reason, negative results with these tests do not rule out infection. Serology testing using specific IgM and neutralizing antibodies is also available that can be used for diagnosis in later stages of the disease.10
STARI: The Other Bull’s-Eye
A common belief is that the appearance of a bull’s-eye rash, also called erythema migrans (Figure 1), around a tick bite is a sign of Lyme disease—but this is not necessarily true. Although the distinctive red rash can indicate Lyme disease, which is caused by the spirochete Borrelia burgdorferi transmitted by the deer tick (Ixodes scapularis), individuals who have been bitten by the lone star tick (Ambylomma americanum) may also develop a bull’s-eye rash. This condition has been named southern tick-associated rash illness (STARI), because the distribution of the lone star tick is in the southeastern United States.11
The causative agent for STARI is still not known. One early study in 2001 examined a patient who had developed a bull’s-eye rash in response to a tick bite while visiting North Carolina but tested negative for Lyme. DNA consistent with a recently identified spirochete, Borrelia lonestari, was found in a punch biopsy taken from the patient’s skin lesion as well as the tick removed from the patient.12 However, a later study demonstrated multiple patients in Missouri and New York whose skin biopsy samples and serological studies found no evidence of exposure to B. lonestari. Each sample from Missouri also showed no exposure to B. burgdorferi, while 50% to 63% of the samples from New York did show some evidence of recent exposure to B. burgdorferi.13
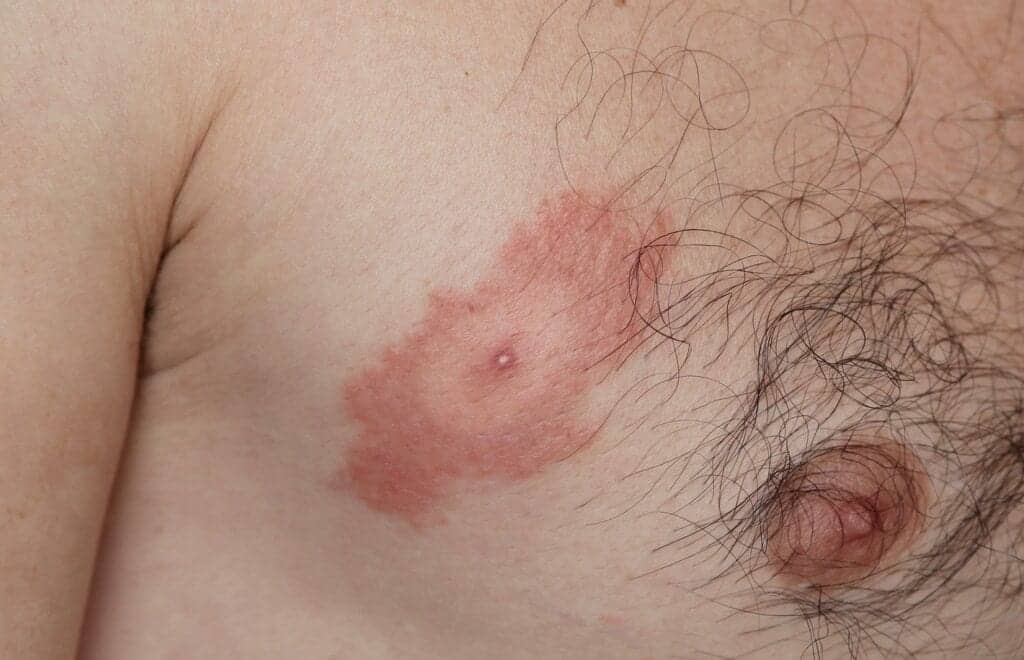
The initial symptoms of STARI mirror Lyme disease. Along with the characteristic rash or skin lesion, patients may also experience fatigue, headache, fever, and muscle pains. These symptoms resolve after treatment with the antibiotic doxycycline. There are some subtle differences between the two illnesses. STARI patients are less likely to experience symptoms other than the rash when compared to individuals with Lyme disease. The skin lesion seen in STARI patients is smaller in size and more circular in shape than the typical bull’s-eye seen with Lyme disease. Additionally, STARI has not been shown to progress to arthritis, neurologic disease, or other chronic symptoms.11
At this time, there are no diagnostic blood tests for STARI because the causative agent is unknown. Patients with STARI will not demonstrate antibodies to B. burgdorferi with traditional Lyme serological tests. This condition is diagnosed based solely on the symptoms and the possibility of a tick bite in an area where Lyme disease is not prevalent. Because STARI does resemble the early stages of Lyme disease, patients can be treated with oral antibiotics as a precautionary measure.11
A Traveler’s Affliction
Borrelia burgdorferi is not the only bacterium in this genus that infects humans. Three other species—B. hermsii, B. parkeri, and B. turicatae—are known to cause an illness called tick-borne relapsing fever (TBRF). The main characteristic of this illness is the recurring pattern of high fever lasting 3 to 5 days, followed by an apparent recovery lasting 5 to 7 days. This pattern can repeat several times unless the patient is treated with antibiotics. The febrile and afebrile intervals are due to the spirochete’s ability to rearrange the molecules present on its outer surface, which causes alternating increases of the individual’s immune system and the total number of spirochetes.14
The three Borrelia species that cause TBRF are transmitted by three separate “soft tick” species that can be found in the western United States. An overall assessment of reported cases performed from 1990 to 2011 found that 70% of all reported cases were contracted in California, Washington, and Colorado. What is unusual about TBRF is that 66% of the cases were reported from nonresident visitors to the place where the individual was exposed.15 This can be explained by the life cycle of the ticks that transmit these organisms.
Unlike the “hard ticks” such as the deer tick, “soft ticks” typically live in the burrows of rodents such as squirrels, chipmunks, and prairie dogs, and will feed on the rodent while it sleeps. Humans are commonly exposed when sleeping in a location infested with rodents, such as a rustic cabin in the mountains. The tick will emerge at night from its nest and feed while the person is sleeping. Because the tick’s bite can be brief, the transmission of the borrelia organism can occur in as little as 30 seconds. “Soft ticks” can live as long as 10 years and go 5 years without feeding, so tick-infested buildings will remain a threat until the nest is removed.14,15
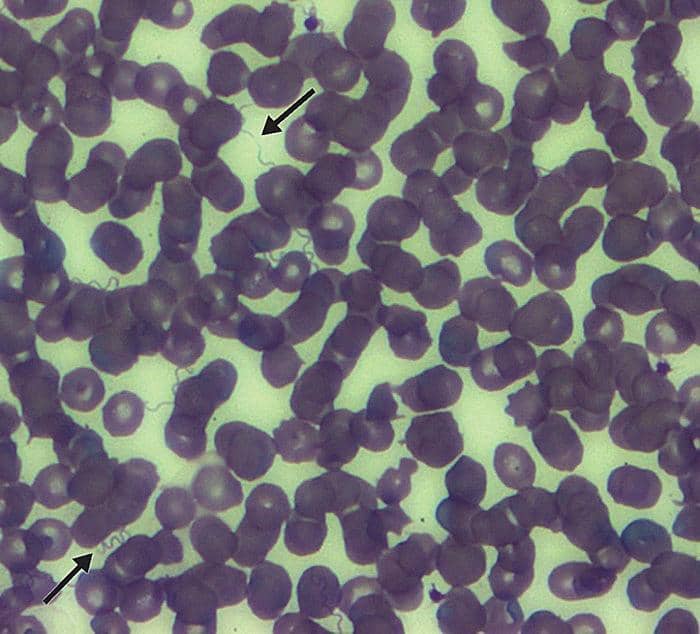
Another unusual characteristic that distinguishes TBRF from Lyme disease is that microscopic examination of stained peripheral blood smears can be used for diagnosis. TBRF patients can demonstrate high concentrations of spirochetes in their blood (≥106 spirochetes/mL), especially if they are untreated and symptomatic (Figure 2). Because the borrelia spirochetes may be confused with other organisms, it is important to take the patient’s clinical presentation and location of potential exposure into consideration. The borrelia species that cause TBRF are also similar enough to B. burgdorferi that TBRF patients may demonstrate positive serology tests for Lyme. A diagnosis of TBRF should be considered for patients with positive Lyme serology tests who have not been in an area where Lyme disease is prevalent. In addition to microscopy, diagnosis can be made with TBRF-specific serology tests as well as culture.14
Red Meat Allergy?
Did you ever think that being bitten by a tick could cause an allergy to red meat? Well, there is some evidence to suggest that it can. The allergy is to alpha-gal (galactose-alpha-1,3-galactose), a sugar molecule that can be found in most mammals with the exception of humans, apes, and monkeys. Most cases have been reported in the southeastern and Midwestern parts of the United States. While a large number of the individuals affected are over 50 years old, children and younger adults can also develop the allergy.16
Alpha-gal allergies were first identified around 2005, when Cetuximab, a monoclonal antibody drug used to treat cancer, was approved for use. It was observed that a large number of patients experienced severe reactions while receiving their first infusion of the new drug. Through several investigations, it was noticed that the reactions occurred in patients in the southeastern United States and that the pattern matched the areas where Rocky Mountain spotted fever is common. Additional studies have concluded that alpha-gal allergies are developed in response to a lone star tick bite and that the drug Cetuximab cross-reacted with the alpha-gal antibody because a portion of the drug antibody has alpha-gal attached.18
During a tick bite, a number of different proteins are injected to help prolong the attachment and improve the ability to feed. One of the proteins that could be injected during the tick bite is alpha-gal, which can stimulate an individual to create IgE antibodies. The allergic response occurs typically 3 to 6 hours after eating red meat because digestion must occur to release the alpha-gal molecules.17 Patients can experience a wide range of symptoms, including hives, a drop in blood pressure, severe stomach pain, and anaphylaxis.16
Blood tests are available to identify and quantify IgE antibodies to alpha-gal. It is important to note that not all individuals who have alpha-gal antibodies present may demonstrate symptoms of the allergy. A 2006 study examined random individuals in Virginia, North Carolina, and Tennessee and found that as many as 20% of the population may have IgE antibodies to alpha-gal, and it is unlikely all those individuals experienced symptoms.
Additionally, studies have followed patients’ total and alpha-gal IgE levels after a tick bite. Some people were identified who had increasing levels of total and specific IgE but had no symptoms of the allergy.18
What’s Next?
For many reasons, including growing human populations and increased travel, control of tick-borne diseases will always be a challenge.19 The habitats of the most common tick species in the United States are expanding, driving the pathogens they carry into new populations.20 But thanks to the work of many scientists and medical professionals and to evolving test methods including next generation sequencing, detecting novel tick-borne bacteria, viruses, and parasites is possible. Rapid identification of novel pathogens allows for control measures to be implemented earlier in a possible outbreak, which increases the effectiveness of the control measures.19

Laboratorians can also play an important role with this process by helping educate caregivers on potential laboratory testing for a sick patient, as well as taking all possible steps in the lab to provide high quality test results.
Nicole Colby is the technical training specialist for COLA, a physician-directed organization whose purpose is to promote excellence in laboratory medicine and patient care through a program of voluntary education, consultation, and accreditation. Colby works to innovate COLA’s technical education and training offerings for staff members and customers.
References
1. Centers for Disease Control and Prevention. Tickborne Disease Surveillance Data Summary. https://www.cdc.gov/ticks/data-summary. Accessed August 18, 2020.
2. Centers for Disease Control and Prevention. Nationally Notifiable Infectious Diseases and Conditions, United States: Annual Tables (2017, 2018). https://wonder.cdc.gov/nndss/nndss_annual_tables_menu.asp. Accessed August 18, 2020.
3. Rosenberg R, Lindsey N, Fischer M, et al. Vital signs: trends in reported vectorborne disease cases – United States and territories, 2004-2016. MMWR Morb Mortal Wkly Rep. 2018;67(17):496-501.
4. McMullan L, Folk S, Kelly A, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834-41. doi:10.1056/NEJMoa1203378.
5. Pastula D, Turabelidze G, Yates K, et al. Notes from the field: Heartland virus disease – United States, 2012-2013. MMWR Morb Mortal Wkly Rep. 2014;63(12):270-271.
6. Centers for Disease Control and Prevention. Heartland virus disease (Heartland). https://www.cdc.gov/heartland-virus. Accessed August 18, 2020.
7. Hermance M, Thangamani S. Powassan virus: an emerging arbovirus of public health concern in North America. Vector Borne Zoonotic Dis. 2017;17(7):453-462. doi:10.1089/vbz.2017.2110.
8. Centers for Disease Control and Prevention. ArboNET Disease Maps. https://wwwn.cdc.gov/arbonet/maps/ADB_Diseases_Map/index.html. Accessed August 20, 2020.
9. El Khoury M, Camargo J, White J, et al. Potential role of deer tick virus in Powassan encephalitis cases in Lyme disease-endemic areas of New York, USA. Emerg Infect Dis. 2013;19(12):1926-1933. doi:10.3201/eid1912.130903.
10. Centers for Disease Control and Prevention. Powassan Virus. https://www.cdc.gov/powassan. Accessed August 18, 2020.
11. Centers for Disease Control and Prevention. Southern tick-associated rash illness. https://www.cdc.gov/stari. Accessed August 19, 2020.
12. James A, Liveris D, Wormser G, et al. Borrelia lonestari infection after a bite by an Amblyomma americanum tick. J Infect. 2001;183:1810-1814. doi:10.1086/320721.
13. Wormser G, Masters E, Liveris D, et al. Microbiologic evaluation of patients from Missouri with erythema migrans. Clin Infect Dis. 2005;40:423-428. doi:10.1086/427289.
14. Centers for Disease Control and Prevention. Tick-borne relapsing fever. https://www.cdc.gov/relapsing-fever. Accessed August 19, 2020.
15. Forrester J, Kjemtrup A, Fritz C, et al. Tickborne relapsing fever – United States, 1990-2011. MMWR Morb Mortal Wkly Rep. 2015;64(03):58-60.
16. Centers for Disease Control and Prevention. Alpha-gal Allergy. https://www.cdc.gov/ticks/alpha-gal. Accessed August 18, 2020.
17. Crispell G, Commins S, Archer-Hartman S, et al. Discovery of alpha-gal-containing antigens in North American tick species believed to induce red meat allergy. Front Immunol. 2019;10:1-16. doi:10.3389/fimmu.2019.01056.
18. Commins S, James H, Kelly E, et al. The relevance of tick bites to the production of IgE antibodies to mammalian oligosaccharide galactose-α-1,3-galactose. J Allergy Clin Immunol. 2011;127(5):1286-1293. doi:10.1016/j.jaci.2011.02.019.
19. Kilpatrick A, Randolph S. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet. 2012;380:1946-1955. doi:10.1016/S0140-6736(12)61151-9.
20. Petersen L, Nasci R, Beard C, Massung R. Emerging vector-borne diseases in the United States: what is next, and are we prepared? Global Health Impacts of Vector-Borne Diseases: Workshop Summary. 2016. https://www.ncbi.nlm.nih.gov/books/NBK390433. Accessed August 26, 2020.