It can take up to six years to adequately diagnose celiac disease. Today, there are an array of non-invasive serological tests that are highly accurate and, more importantly, can help cut the time to diagnosis.
By Aya Elhage, PharmD, MBA; Ilana Heckler, PhD; Iswariya Venkataraman, PhD
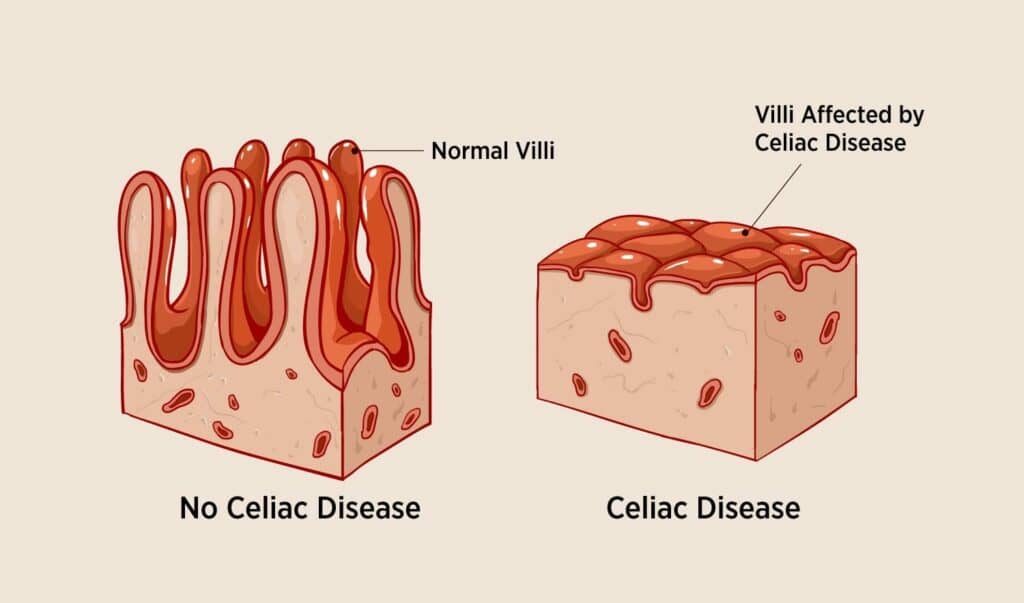
Celiac disease (CD) is an immune-mediated systemic enteropathy triggered by gluten consumption leading to damage of the gastrointestinal (GI) mucosa, particularly the small intestine.1 (Figure 1) Gluten and related prolamins are the predominant proteins found in wheat, barley, and rye. Gluten is a major environmental factor that triggers CD in genetically predisposed individuals.2
The clinical manifestations of CD can be generalized, making it quite difficult to know when to seek diagnostic testing. Symptoms may mimic those of other conditions that impact the GI mucosa such as Crohn’s disease, irritable bowel syndrome, and lactose intolerance among others.3 Common symptoms may include diarrhea, abdominal pain, constipation, bloating and distention, unexplained weight loss, and fatigue.2
It is estimated that CD affects 1% of the world’s population, with a higher prevalence in females.4,5Furthermore, in the United States, over 2.5 million Americans remain undiagnosed which could increase their chances of developing another autoimmune condition by about 20%.4
Pathogenesis of CD
Gluten contains two major proteins, gliadins and glutenins, that are the major known environmental factors required for CD activation.6 Additionally, CD is often found in genetically susceptible individuals carrying specific human leukocyte antigen (HLA) genes including HLA-DQ2 and DQ8 in >90% and >5% of cases, respectively.1,7Therefore, both environmental factors and genetic predisposition (HLA-DQ2/DQ8 positivity) are deemed mandatory for the development of CD.1 Thus, in patients negative for both DQ2 and DQ8 alleles, CD can be virtually excluded and no further testing is required (≥95% negative predictive value).2
After the ingestion of gluten, gliadin peptides are translocated through the gastric epithelium into the lamina propria where they are deaminated by tissue transglutaminase (tTG) into glutamic acid.8 These deamidated gliadin peptides (DGP) are presented to pathogenic CD4+ T cells on antigen-presenting cells positive for DQ2 or DQ8.8 Once DGP is bound to DQ2 or DQ8, several proinflammatory cytokines are produced, which triggers an immune reaction resulting in production of anti-tTG and anti-DGP antibodies.8
Diagnostic Criteria & Differential Diagnosis of CD
Although this disease can occur at any age, CD most frequently occurs in pediatrics and tends to occur early on within the first 2 years of life.9 According to Daniel Leffler, MD, MS, from the Celiac Center at Beth Israel Deaconess Medical Center, it takes approximately 6-10 years for an individual to be accurately diagnosed with CD.10Therefore, any delay in diagnosis or treatment of CD can lead to long-term complications such as iron deficiency anemia, early onset osteoporosis, infertility, or GI cancers.4 Due to its hereditary nature, there is a 10% chance that first-degree relatives will develop CD in the presence of these HLA genes.4
Before the updated guidelines by the American College of Gastroenterology (ACG) and European Society of Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) published in 2013 and 2020 respectively, CD diagnosis relied heavily on duodenal biopsies. Since then, clinical practice has shifted to a no-biopsy approach with a strong recommendation of using serological testing of CD-specific antibodies as the initial diagnostic method.11,12
Per ACG and ESPGHAN guidelines, immunoglobulin A (IgA) tTG is the superior single test for CD detection in patients above 2 years of age. If IgA is low or not detected, an IgG-based test (i.e., anti-DGP testing) can be utilized. Moreover, HLA genotypic testing can be an effective method to rule out CD in certain clinical situations. If an individual is not positive for DQ2 and DQ8 alleles, the likelihood of a CD diagnosis is low.11 Due to the lack of standardization and high cost of genotypic testing, alternative rapid and cost-efficient tests are crucial to provide new insights on the utility of HLA typing to aid in the diagnosis and management of CD.
CD-specific Antibodies and Detection Techniques
The detection of antibodies against tTG represents both a highly specific and sensitive method for CD. This is due to the high prevalence of IgA antibodies against tTG found in CD patients, and the absence of such antibodies in healthy individuals. Anti-tTG antibodies can be detected using monospecific immunoassays such as ELISAs, immunoblots, or indirect immunofluorescence tests (IIFT) that utilize transfected cell substrates having recombinantly expressed tTG. tTG is also the target antigen of anti-endomysial antibodies (EMA).13 EMA can be detected by IIFT using liver or monkey esophagus tissue. Due to high sensitivity, anti-tTG IgA ELISAs have gradually replaced testing for EMA in the serological diagnosis of CD.13
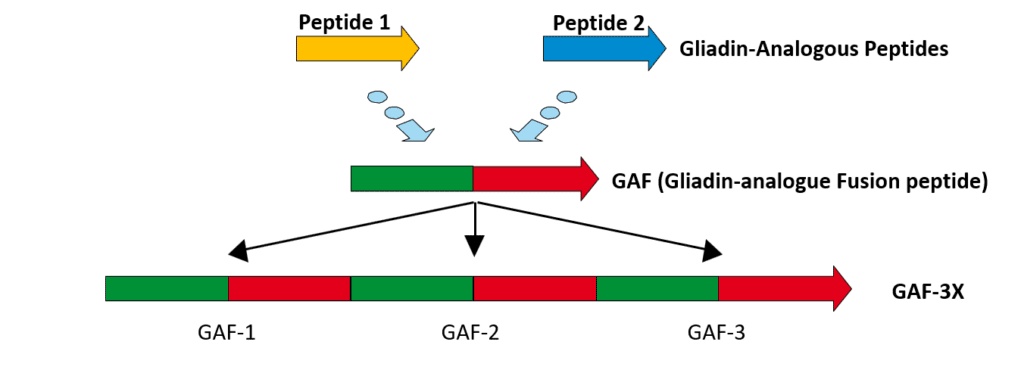
Antibodies against native gliadin are no longer considered to be suitable markers for CD as they also are found in healthy individuals and patients with other disorders such as schizophrenia and psoriasis.14,15 When used as the antigenic target for ELISA, native gliadin has a low sensitivity (58% for IgA and 53% for IgG antibodies).16 However, deamidated epitopes of gliadin fragments represent a specific target and have been recognized as a suitable marker for CD.17 As only a select number of deamidated epitopes of gliadin are diagnostically relevant, a gliadin-analogous fusion (GAF) peptide has been developed in a trimeric form (3X) which increases the diagnostic sensitivity of the ELISA to 84% for IgA and 80% for IgG when used as an antigenic target.16 (Figure 2) This sensitivity for the GAF-3X IgG ELISA is comparable to that of the anti-tTG IgA ELISA, which is 78%.16
The parallel determination of both anti-GAF-3X IgG and anti-tTG IgA antibodies achieves the highest diagnostic accuracy. The combination of anti-GAF-3X IgG and anti-tTG IgA detection shows the highest serological hit rate for CD (93%).16 The EUROLINE (EUROIMMUN, Germany) Celiac Disease Profile immunoblot allows for the parallel detection of anti-tTG and anti-GAF-3X antibodies, with an additional control for IgA deficiency. Sensitivities for IgA and IgG GAF-3x antibodies were 88.9% with specificities of 97.0% and 100.0%, respectively. For anti-tTG, the sensitivities for IgA and IgG were 100.0% at specificities of 96.9% and 100.0%, respectively. Guidelines recommend the determination of anti-tTG IgA or deaminated gliadin peptide IgA every 3-6 months in the first year after CD diagnosis and every year thereafter to monitor the disease.12 In CD patients with IgA deficiency (which is estimated to occur in 2% of CD patients), the detection of deaminated gliadin peptide IgG, and tTG IgG antibodies is recommended.12 In other unique populations such as children (< 2 years), early stage CD, CD-associated dermatitis herpetiformis, and for monitoring dietary compliance, antibodies to DGP were found to be superior to tTG.18,19
Monitoring a Gluten-Free Diet through CD-specific Antibodies
CD can only be treated by adhering to a gluten-free diet (GFD). Under this lifelong diet, CD-specific antibody titers decrease.20 Therefore, a GFD can be monitored through the detection of antibodies to GAF-3X and tTG. In CD patients following a GFD, antibodies should decrease significantly within 6 months and reach normal levels within the first year.20 Therefore, continued antibody positivity can indicate that a person is not adherent to a GFD.9 Patients with CD whose antibody titers do not normalize are recommended to be reassessed for gluten exposure. Patients can meet with dietitians to revise their diets to exclude possible gluten contamination.
Genetic Susceptibility for CD: HLA-DQ2 & DQ8
The genetic risk for CD is related to specific HLA DQ2 and DQ8. HLA-DQ2 and/or -DQ8 markers are present in >98% of CD patients.21 The detection of HLA-DQ2 and -DQ8 markers are particularly important in asymptomatic patients having a family history of CD or other autoimmune diseases. The absence of HLA-DQ2 and -DQ8 markers, due to their high negative predictive value, allows for the exclusion of CD if both markers are not detected. In symptomatic patients, HLA-DQ2 and -DQ8 can be used to confirm CD. Additionally, the detection of these genetic biomarkers can be used to confirm CD in patients with inconclusive biopsy or serology results. For example, in cases where CD patients on a gluten-free diet, who therefore have very low CD-specific antibody titers, genetic testing is a useful option.
Using DNA microarray solutions such as the EUROArray system (EUROIMMUN, Germany), the identification of the disease-associated alleles HLA-DQA1- and HLA-DQB1, which code for the subunits HLA-DQ2.2, -DQ2.5 and -DQ8, can be done. This test can differentiate between the homozygous and heterozygous presence of the alleles which code the alpha and beta subunits of HLA-DQ2.2 and -DQ2.5 which enables improved risk assessment in the case of a positive HLA-DQ2 result.
Future Perspectives
An accurate and early detection of CD is critical as cases of undiagnosed CD are more likely to develop serious illnesses including osteoporosis, dermatitis herpetiformis, chronic fatigue, thyroiditis, and other autoimmune diseases.1 Serological and genetic testing can be used to help avoid a misdiagnoses and therefore prevent an unjustified gluten-free diet. Additionally, serological testing is useful for the monitoring of disease activity in celiac patients.
Even though it is not part of the diagnostic guidelines, clinicians should detect in parallel both anti-GAF-3X IgG and anti-tTG IgA antibodies to achieve the highest diagnostic accuracy (93% for CD).16 As the current treatment for CD is a gluten-free diet, serological testing allows for the determination of GFD compliance. Due to the restrictiveness and difficulty to follow a GFD, continued research to discover new therapeutics and preventative strategies is needed to improve the quality of life and find a cure for CD patients. The development of home-based CD testing allows for a simple way to test yourself or family member at home which can help increase diagnosis rates.
Featured illustration: The Authors
ABOUT THE AUTHORS
Aya Elhage, PharmD, MBA, is a pharmacist with a passion for bridging the gap between diagnostics and therapeutics to transform the world of healthcare. Elhage currently serves as the scientific affairs manager at EUROIMMUN US, a medical diagnostics company, supporting the team with commercial activities including scientific collaborations, scientific marketing, and business development of diagnostics. She is also a registered pharmacist in both New York and California.
Iswariya Venkataraman, PhD, is the associate director of the Scientific Affairs team at EUROIMMUN US. In this role, she builds scientific partnerships with healthcare professionals and key decision makers through evidence-based and non-promotional scientific activities. She holds a PhD in neuroscience and during her studies she identified novel autoantigens in autoimmune hyper-excitability disorders. She has written several scientific articles regarding biomarkers in neurodegeneration and her work has been published in high impact journals such as Alzheimer’s & Dementia.
Ilana Heckler, PhD, is the scientific affairs associate at EUROIMMUN US. She holds a PhD in chemical biology for her studies on bacterial hemoprotein sensors of nitric oxide. As the scientific affairs associate, Heckler supports scientific collaborations and assists in the validation of diagnostic assays for autoimmune and infectious diseases.
REFERENCES
1. Caio G, Volta U, Sapone A, et al. Celiac disease: a comprehensive current review. BMC Medicine. 2019;17(1):142.
2. Hill ID, Fasano A, Guandalini S, et al. NASPGHAN Clinical Report on the Diagnosis and Treatment of Gluten-related Disorders. Journal of Pediatric Gastroenterology and Nutrition. 2016;63(1):156-165.
3. Celiac B. CELIAC DISEASE TESTING AND DIAGNOSIS. Getting Tested Web site. https://www.beyondceliac.org/celiac-disease/get-tested/. Accessed July 15, 2021, 2021.
4. Foundation CD. What is Celiac Disease? About Celiac Disease Web site. https://celiac.org/about-celiac-disease/what-is-celiac-disease/. Accessed July 14, 2021.
5. Megiorni F, Mora B, Bonamico M, et al. HLA-DQ and susceptibility to celiac disease: evidence for gender differences and parent-of-origin effects. Am J Gastroenterol. 2008;103(4):997-1003.
6. Kagnoff MF. Overview and pathogenesis of celiac disease. Gastroenterology. 2005;128(4):S10-S18.
7. Fasano A. Celiac disease–how to handle a clinical chameleon. N Engl J Med. 2003;348(25):2568-2570.
8. Ciclitira PJ, Johnson MW, Dewar DH, Ellis HJ. The pathogenesis of coeliac disease. Mol Aspects Med. 2005;26(6):421-458.
9. Parzanese I, Qehajaj D, Patrinicola F, et al. Celiac disease: From pathophysiology to treatment. World J Gastrointest Pathophysiol. 2017;8(2):27-38.
10. Celiac B. CELIAC DISEASE: FAST FACTS. https://www.beyondceliac.org/celiac-disease/facts-and-figures/. Accessed July 20, 2021.
11. Husby S, Koletzko S, Korponay-Szabó I, et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. Journal of Pediatric Gastroenterology and Nutrition. 2020;70(1):141-156.
12. Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA. ACG Clinical Guidelines: Diagnosis and Management of Celiac Disease. Official journal of the American College of Gastroenterology | ACG. 2013;108(5):656-676.
13. Tonutti E, Bizzaro N. 55 – ANTI-TISSUE TRANSGLUTAMINASE AND ANTI-ENDOMYISIAL ANTIBODIES. In: Shoenfeld Y, Gershwin ME, Meroni PL, eds. Autoantibodies (Second Edition). Burlington: Elsevier; 2007:443-450.
14. Jin S-Z, Wu N, Xu Q, et al. A study of circulating gliadin antibodies in schizophrenia among a Chinese population. Schizophr Bull. 2012;38(3):514-518.
15. Michaëlsson G, Gerdén B, Ottosson M, et al. Patients with psoriasis often have increased serum levels of IgA antibodies to gliadin. Br J Dermatol. 1993;129(6):667-673.
16. Kasperkiewicz M, Dähnrich C, Probst C, et al. Novel assay for detecting celiac disease–associated autoantibodies in dermatitis herpetiformis using deamidated gliadin-analogous fusion peptides. Journal of the American Academy of Dermatology. 2012;66(4):583-588.
17. Aleanzi M, Demonte AM, Esper C, Garcilazo S, Waggener M. Celiac disease: antibody recognition against native and selectively deamidated gliadin peptides. Clin Chem. 2001;47(11):2023-2028.
18. Kurppa K, Lindfors K, Collin P, et al. Antibodies against deamidated gliadin peptides in early-stage celiac disease. J Clin Gastroenterol. 2011;45(8):673-678.
19. Arigliani M, Rech Morassutti F, Fabris M, Melli P, Tonutti E, Cogo P. Coeliac disease in infants: antibodies to deamidated gliadin peptide come first! Ital J Pediatr. 2017;43(1):70-70.
20. Midhagen G, Åberg A-K, Olcén P, et al. Antibody levels in adult patients with coeliac disease during gluten-free diet: a rapid initial decrease of clinical importance. Journal of Internal Medicine. 2004;256(6):519-524.
21. Cecilio LA, Bonatto MW. The prevalence of HLA DQ2 and DQ8 in patients with celiac disease, in family and in general population. Arq Bras Cir Dig. 2015;28(3):183-185.