Component-resolved diagnostics gauge food allergies from asymptomatic to life-threatening
By Robert W. Reinhardt, MD
Peanuts are the most common cause of fatal food anaphylaxis.1 In a 2008 study, the estimated prevalence of clinical peanut allergy among children in the United States was 1.4%.2 Many more (up to 11.8%) may be sensitized to whole peanut (having a positive in vitro s-IgE or positive skin test)3 but may not not experience a systemic response to an oral food challenge (OFC) with peanut. To further complicate the picture, multiple studies have shown that 50% to 90% of presumed food allergies are not actually allergies (that is, IgE-mediated) at all.4
These facts illustrate the dilemma facing clinicians and their patients: Patient histories are often unclear, and sensitization alone does not necessarily predict risk for severe systemic reactions. Similar issues exist for milk, egg, soy, and wheat—the foods that account for most of the other identified food allergies in childhood. The concern is pertinent because some food reactions are severe, persist for a lifetime, and must be managed with strict dietary elimination and immediate access to epinephrine in case of accidental ingestion. On the other hand, a child likely to “outgrow” food sensitivity (ie, become tolerant), or who may be at low risk for a systemic reaction, shouldn’t be burdened with dietary anxiety or unnecessary lifestyle restrictions.
A food allergy is defined as “an adverse health effect arising from a specific IgE-mediated immune response that occurs reproducibly on exposure to a given food.”4 Food allergens are the specific components—typically, proteins—recognized by immune cells that elicit specific IgE (s-IgE) reactions and characteristic symptoms. The whole-allergen extracts traditionally used for diagnostic testing (both in vitro s-IgE and skin prick testing) contain all the extractable proteins present in the source material, some highly allergenic and some not. A patient may be sensitized to an allergen extract, meaning IgE is produced, but not allergic to that allergen, meaning clinical symptoms are not associated with the presence of s-IgE. A positive s-IgE result to the whole allergen indicates sensitization, but cannot pinpoint exactly which specific protein components in that extract are responsible for the sensitization. However, component-resolved diagnostics (CRD) complements whole-extract testing by identifying the specific components in the extract that are associated with sensitization, differentiating between primary or species-specific components, as well as secondary markers of cross-reactivity. This, in turn, helps the physician evaluate the risk of clinical reactivity on exposure to a potentially allergenic source.
This article explores the technology behind CRD and demonstrates how laboratory test results can be used to help the physician assess risk for allergic reactions, evaluate potential tolerance, and optimize management of food allergies in their allergenic patients.
THE SCIENCE BEHIND COMPONENT TESTING
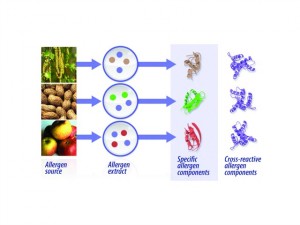
Figure 1. From allergen source to components. Specific allergen components can be separated and purified from natural allergen sources or produced using recombinant technology. Sensitization to specific or cross-reactive allergen components can then be measured individually in separate tests.
An allergenic source may contain dozens of individual specific proteins, only some of which are allergenic and capable of causing clinical disease (Figure 1). No common features identify the many protein components in a single source as allergenic. Some IgE antibodies formed to these components bind only to that particular protein. However, proteins with similar structures are often found in biologically related or homologous species, and IgE antibodies formed against these structures can bind to the same or closely related structures on a protein from multiple species. This is called “cross-reactivity.”
Allergen components are identified by the first three letters of the genus name and the first letter of the species name, followed by a number. For example, peanut is Arachis hypogeae, and so peanut components are labeled Ara h 1, Ara h 2, Ara h 3, etc. The value of identifying species-specific allergen components is that it reveals the primary sensitizer(s) that are associated with a whole-allergen source, such as peanut.
Other allergen components are classified as markers for cross-reactivity due to their similar protein structures to other allergens and IgE-binding properties. They can be present in many different allergenic sources, sometimes only distantly related to a suspected allergenic source. A good example of cross-reactivity can be illustrated by birch pollen-related food allergy, a syndrome affecting many Scandinavian patients who are exposed to birch pollen and become sensitized. On the molecular level, most patients who are allergic to birch have IgE antibodies to Bet v 1, which is structurally related to proteins in soy, peanut, apple, latex, and hazelnut. Thus, the patient’s IgE antibodies to birch Bet v 1 may cross-react with proteins in foods that may share a similar, or homologous, structure.
[reference id=”37500″]Table 1[/reference]
Plant food proteins are grouped into families depending on their structure and function. The most studied plant food protein families are described in Table 1. Knowing to which allergen family a component belongs helps in understanding the possible associated clinical symptoms. For example, proteins that belong to the storage protein family are primarily species-specific, known to be stable to heat and digestion, and are associated with more severe clinical symptoms. In contrast, proteins belonging to the PR-10 group are spread across the plant kingdom and are highly homologous. They are generally recognized as cross-reactive proteins, are heat- and digestion-labile, and are typically associated with milder clinical symptoms.
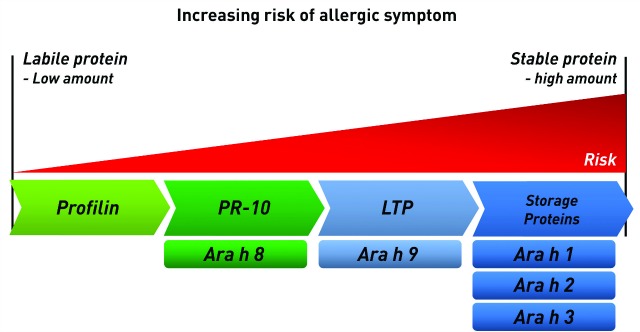
Figure 2. Allergic risk associated with protein families. Certain food protein classes, such as storage proteins, are more likely to cause clinical symptoms. Stable food proteins are also likely to cause more severe symptoms and reactions than labile proteins. Thus, sensitization to peanut components Ara h 8 and Ara h 9, both lipid transfer proteins (LTPs), poses less risk than sensitization to storage proteins Ara h 1, Ara h 2, and Ara h 3.
Protein stability also affects the allergenicity of the protein itself. Allergen components that are stable to heat and digestion are more likely to cause a severe systemic reaction because these proteins reach the systemic circulation in largely intact form. Heat and digestion-labile allergens, which are easily broken down by processing, cooking, or enzymes in the saliva and gut, are more likely to be tolerated or only cause mild/local symptoms (Figure 2, page 14). Oral allergy syndrome (OAS), for example, is a mild to moderate oral contact urticaria that occurs in patients sensitized to panallergens, such as PR-10 proteins.5
Protein stability may affect a person’s ability to tolerate different foods and influence the severity of clinical reactions. Some food allergens may cause reactions when they are consumed raw or have not been fully cooked, but those same allergens may no longer be allergenic after they have been thoroughly cooked. Some allergens will give rise to clinical reactions ranging from mild or moderate to severe, whereas others will cause sensitization without a clinical reaction.6 One caveat applies: The ingested dose of allergen affects outcome. Even rather labile proteins may potentially give rise to systemic reactions if the quantities are sufficient.
THE FIRST FDA-CLEARED COMPONENT TESTING
The first CRD assays cleared by FDA were for components of milk and egg (Thermo Fisher, ImmunoCAP Allergen Components). Today the company’s offerings include more than 50 allergen components, in addition to the more than 300 whole-extract allergens, together comprising the largest FDA-cleared allergen test menu.7 The allergen components utilize the proven ImmunoCAP technology, with reliable quantitative reporting of s-IgE down to 0.1 KUA/L (limit of quantitation). Allergen component testing is performed on the same automated instrument systems as whole-extract allergen assays (Phadia Laboratory Systems), providing walk-away productivity for tests categorized as moderate complexity under the terms of the Clinical Laboratory Improvement Amendments of 1988 (CLIA). This assay technology has been referenced or used in more than 4000 scientific publications and acknowledged in National Institutes of Health and international guidelines.4,6,7
Extensive published studies performed on the allergen components have helped to characterize the significance of IgE antibodies to individual components. The results of component-level testing support a molecular-level understanding of the patient’s individual constellation of symptoms, and can aid in the diagnosis of these IgE-mediated allergic disorders. Individualized actions could include targeted food avoidance, the advisability and risk associated with performing clinician-supervised OFC, or selection for specific immunotherapy (SIT).
Allergen component testing may be especially useful given the barriers to conducting OFCs, which include referral to an allergy specialist, the risk of an adverse event, and cost to the
healthcare system.8
COMPONENT-RESOLVED DIAGNOSTICS IN PRACTICE
Peanut is one of the most allergenic foods, capable of eliciting acute and often severe allergic reactions. The first clinical reactions to peanut typically occur before age 2 and seldom after age 17, and in more than 70% of patients a reaction resulted from the first exposure to peanut.9 At least eight of the more than 30 proteins in peanuts can bind IgE, and the major allergenic components of peanut release easily in the mouth and resist thermal, chemical, and proteolytic denaturation.10 Whole peanut s-IgE concentrations >15 KUA/L have long been considered >95% predictive of clinical reactions and may by itself provide sufficient evidence to eliminate the need to perform an OFC.11
Studies have demonstrated that sensitization to certain peanut storage-protein components (Ara h 1, Ara h 2, and Ara h 3) greatly increases the risk of systemic reaction, and sensitization to Ara h 2 is nearly always associated with clinical peanut allergy.12 However, patients sensitized to Ara h 8 alone may face a low risk of systemic reaction12 but may experience localized itching/tingling of the lips, mouth, and oropharynx.13 Results for Ara h 9 must be correlated with any known symptomatic history. If there is no history of clinical symptoms, the risk is low, but in the presence of clinical symptoms, Ara h 9-sensitized patients should be managed with the same precautions as children with confirmed peanut allergy. Both Ara h 8 and Ara h 9 are cross-reactive with pollens13 and pitted fruits, respectively.14 Geographical variations in peanut component sensitization have been documented, with Americans frequently sensitized to Ara h 1, Ara h 2, and Ara h 3, while patients in Spain and Sweden were more likely sensitized to Ara h 9 and Ara h 8.15
[reference id=”37502″]Table 2[/reference]
The added value of component testing can be seen in patients with low whole-peanut s-IgE concentrations but sensitization to Ara h 2 (Table 2). In the context of a symptomatic history and physical examination, this result suggests a high probability of clinical peanut allergy. Management considerations would include establishing peanut-free zones for the patient’s safety, prescription of an epinephrine auto-injector, and alerting family and teachers to the child’s peanut allergy.
THE HOPE OF SPECIFIC IMMUNOTHERAPY
Specific immunotherapy holds out the hope of gradual desensitization for many peanut-allergic patients. Several small but well-controlled studies using double-blind placebo-controlled food challenges have substantiated the efficacy of oral immunotherapy in patients with peanut allergy. In the STOP II study, 62% of patients (24/39) were considered desensitized, able to ingest as much as 25.5 times more peanut protein after treatment.16 Quality-of-life scores improved significantly and in most patients side effects were mild, consisting primarily of gastrointestinal upset. In a second multicenter study, initial escalation, build-up, and maintenance phases of oral immunotherapy were followed by OFC after a year of treatment.17
There were 19 children in the treatment group and nine in the placebo group; three children dropped out of the treatment group due to allergic side effects. The remaining 16 all ingested the maximum cumulative peanut dose (5,000 mg) during the OFC, equivalent to about 20 peanuts. Placebo patients ingested a dose of only 280 mg. In another multicenter study, 70% of subjects (14/20) treated with sublingual immunotherapy were desensitized by 44 weeks compared to only 15% of subjects receiving placebo.18 Of 10,855 peanut doses administered, 63.1% precipitated no symptoms and, excluding oral/pharyngeal symptoms, 95.2% were symptom-free. In another year-long study of sublingual therapy, the treated group safely ingested 20 times more peanut protein than the untreated group.19 These encouraging results have spurred additional research and may expand SIT from allergy research settings into clinical practice.
CONCLUSION
Component-resolved diagnostic testing is already adding valuable information about patients’ individual sensitivities that, when combined with history and physical examination, may be valuable in clinical decision-making. CRD helps to identify various phenotypes of allergic populations and adds specificity to what are already highly sensitive whole-extract s-IgE tests. Especially noteworthy is the ability to help assess the risk associated with sensitization to specific components, which can help answer the question: Is exposure likely to be asymptomatic or life-threatening? The continuing study of SIT holds out the hope of treatment for many food-allergic patients in the future.
For more information, visit the sidebar “More Component-Resolved Diagnostics: The Case of Milk and Eggs.”
Robert W. Reinhardt, MD, is chief medical officer at Thermo Fisher Scientific Immunodiagnostics, Kalamazoo, Mich. For further information, contact chief editor Steve Halasey via [email protected].
REFERENCES
1. Keet CA, Wood RA. Food allergy and anaphylaxis. Immunol Allergy Clin North Am. 2007;27(2):193–212.
2. Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125(6):1322–1326.
3. Nicholaou N, Poorafshar M, Murray C, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125(1):191–197.
4. Boyce JA, Assa’ad A, Burks AW, et al. NIH Guidelines for the Diagnosis and Management of Food Allergy in the United States: Report of the NIAID Sponsored Expert Panel. NIH publication no. 11–7700, 2010. Available at: http://www.niaid.nih.gov/topics/foodallergy/clinical/documents/faguidelinesexecsummary.pdf. Accessed April 11, 2014.
5. Kazemi-Shirazi L, Pauli G, Purochit A, et al. Quantitative IgE inhibition experiments with purified recombinant allergens indicate pollen-derived allergens as the sensitizing agents responsible for many forms of plant food allergy. J Allergy Clin Immunol. 2000;105(1 pt 1):116–125.
6. Data on file. Thermo Fisher Scientific Inc.
7. Johansson SG. ImmunoCAP specific IgE test: an objective tool for research and routine allergy diagnosis. Expert Rev Mol Diagn. 2004;4(3):273–279.
8. Pongracic JA, Bock SA, Sicherer SH. Oral food challenge practices among allergists in the United States. J Allergy Clin Immunol. 2012;129(2):564–566.
9. Sicherer SH, Burks AW, Sampson HA. Clinical features of acute allergic reactions to peanut and tree nuts in children. Pediatrics. 1998;102(1):E6.
10. Lalles JP, Peltre G. Biochemical features of grain legume allergens in humans and animals. Nutr Rev. 1996;54(4 pt 1):101–107.
11. Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 1997;100(4):444–451.
12. Asarnoj A, Nilsson C, Lidholm J, et al. Peanut component Ara h 8 sensitization and tolerance to peanut. J Allergy Clin Immunol. 2012;130(2):468–472.
13. Mittag D, Akkerdaas J, Ballmer-Weber BD, et al. Ara h 8, a Bet v 1-homologous allergen from peanut, is a major allergen in patients with combined birch pollen and peanut allergy. J Allergy Clin Immunol. 2004;114(6):1410–1417.
14. Lauer I, Dueringer N, Pokoj S, et al. The non-specific lipid transfer protein, Ara h 9, is an important allergen in peanut. Clin Exp Allergy. 2009;39(9):1427–1437.
15. Vereda A, van Hage M, Ahlstedt S, et al. Peanut allergy: clinical and immunologic differences among patients from 3 different geographic regions. J Allergy Clin Immunol. 2011;127(3):603–607.
16. Anagnostou K, Islam S, King Y, et al. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet. 2014 Jan 29. pii: S0140–67 doi: 10.1016/S0140–6736(13)62301–6. [Epub ahead of print]
17. Varshney P, Jones SM, Scurlock AM, et al. A randomized controlled study of peanut oral immunotherapy (OIT): clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127(3):654–660.
18. Fleischer DM, Burks AW, Vickery BP, et al, for the Consortium of Food Allergy Research (CoFAR). Sublingual immunotherapy for peanut allergy: a randomized double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol. 2013;131(1):199–127.
19. Kim EH, Bird JA, Kulis M, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011;127(3):640–646.





