Johns Hopkins researchers develop multi-analyte assay combining tumor DNA, mutations, and immune cell signatures for central nervous system cancer diagnosis.
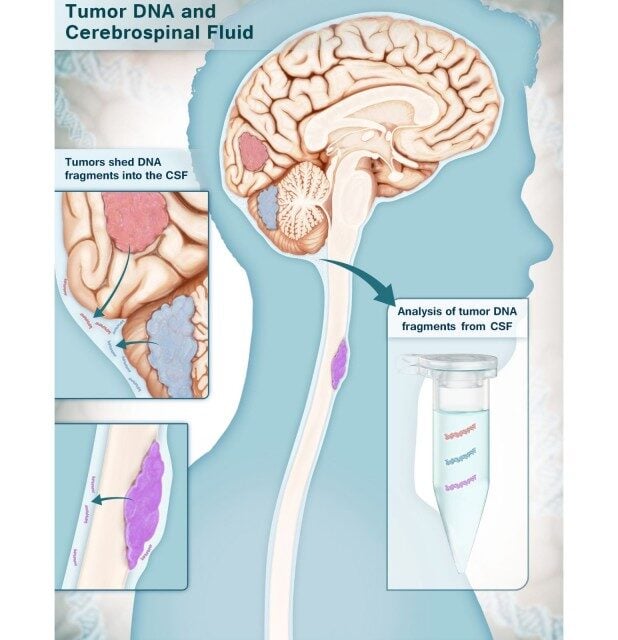
Researchers at Johns Hopkins Kimmel Cancer Center have developed a multi-analyte test that can accurately identify brain cancers using small cerebrospinal fluid (CSF) samples, achieving more than 80% sensitivity and 100% specificity in validation studies.
The test, called CSF-BAM (cerebrospinal fluid–B/T cell receptor, aneuploidy and mutation), combines multiple biological markers including tumor-derived DNA, chromosomal abnormalities, tumor-specific mutations, and T and B cell receptor sequences. The findings were published Aug 25 in Cancer Discovery.
“This study highlights how much more information we can gain when we evaluate several analytes together,” says senior study author Chetan Bettegowda, MD, PhD, Harvey Cushing Professor and director of the Department of Neurosurgery at Johns Hopkins University School of Medicine, in a release. “The ability to detect cancers with high specificity and also gain insight into the immune environment of the brain could be an important advance in the care of patients with brain tumors.”
Validation Across Multiple Cancer Types
The research team analyzed 206 CSF samples from patients with high-grade gliomas, medulloblastomas, metastases, and central nervous system lymphomas. The 100% specificity means no false positives were recorded among individuals with noncancerous conditions in the validation cohort.
The assay also demonstrated the ability to distinguish between immune cell populations present in cancer and noncancer cases, providing additional biological context for challenging clinical scenarios. This capability to categorize T and B cell populations in CSF offers insights into both disease presence and immune response.
“Many patients with brain lesions face invasive diagnostic procedures to confirm a cancer diagnosis,” says Christopher Douville, MD, assistant professor of oncology and senior study author, in a release. “A tool like this could help us make better-informed decisions about who really needs a biopsy and who doesn’t.”
Clinical Applications for Laboratory Diagnostics
The multi-analyte approach could prove particularly valuable for cases where conventional imaging or cytology results are inconclusive, or when obtaining tissue for diagnosis presents risks or is not feasible. The test’s ability to provide both cancer detection and immune environment characterization supports more tailored patient care approaches.
The research demonstrates that combining multiple biological markers is more effective for diagnosing central nervous system cancers than using any single marker alone, potentially offering laboratories a new tool for clinical decision-making support.
The study was supported by National Institutes of Health funding and involved researchers from the Johns Hopkins Ludwig Center and Department of Neurosurgery, along with multiple collaborating institutions.
Photo caption: Multi-analyte test can accurately identify brain cancers using small samples of cerebrospinal fluid.
Photo credit: Elizabeth Cook