PATHFINDER 2 study results indicate more than half of cancers detected by Galleri test were found in early stages, with three-quarters having no current screening options.
Results from the PATHFINDER 2 study show that adding GRAIL’s Galleri multi-cancer early detection blood test to standard cancer screenings increased cancer detection rates more than seven-fold compared to recommended screenings alone.
The prospective study, presented at the European Society for Medical Oncology Congress 2025, evaluated 35,878 enrolled participants across the US and Canada. Results were reported from a pre-specified analysis of the first 25,578 participants with at least 12 months of follow-up as of Dec 31, 2024.
When added to US Preventive Services Task Force A and B recommended screenings for breast, cervical, colorectal, and lung cancers, the Galleri test led to a more than seven-fold increase in cancers detected within one year. The test detected approximately three times as many cancers when added to standard screening for breast, cervical, colorectal, lung, and prostate cancers.
“Adding Galleri to recommended screening for breast, cervical, colorectal, and lung cancers in PATHFINDER 2 yielded a more than seven-fold increase in the cancer detection rate, and more than half of the Galleri detected new cancers were found in early stages, when cancers are more treatable and potentially even curable,” says Josh Ofman, MD, MSHS, president at GRAIL, in a release.
Performance and Safety Results
The Galleri test detected a cancer signal in 216 participants, representing a cancer signal detection rate of 0.93%. Of those, cancer was diagnosed in 133 participants for a cancer detection rate of 0.57%. The positive predictive value was 61.6%.
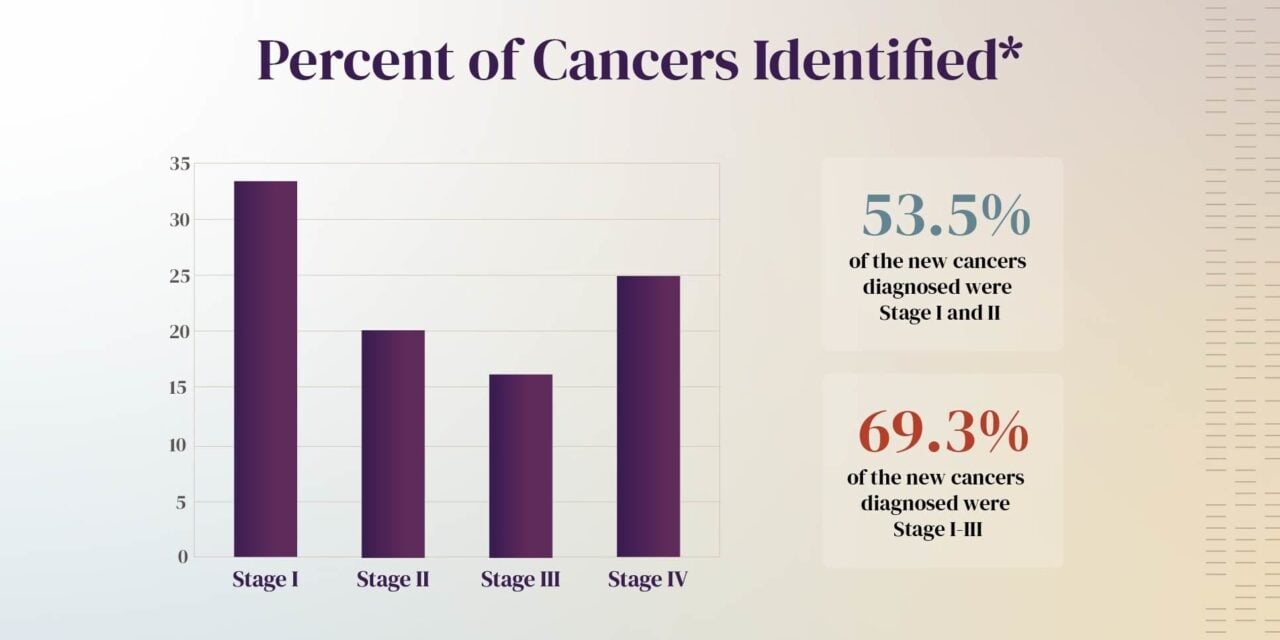
More than half (53.5%) of new cancers detected by Galleri were stage I or II, and more than two-thirds (69.3%) were detected at stages I-III. Approximately three-quarters of the cancers detected by Galleri do not have standard screening options available.
The test demonstrated 73.7% episode sensitivity for the 12 cancers responsible for two-thirds of cancer deaths in the US. For all cancers, episode sensitivity was 40.4%. Specificity was 99.6%, translating to a false positive rate of 0.4%.
The test correctly identified the cancer signal origin 92% of the time, leading to efficient diagnostic workups with a median resolution time of 46 days. Only 0.6% of all participants required an invasive procedure during diagnostic workup.
Regulatory Pathway
GRAIL plans to submit data from PATHFINDER 2 to the FDA as part of the Galleri premarket approval application, along with data from the NHS-Galleri trial. The company will also submit a bridging analysis comparing the study version of Galleri to the updated version planned for FDA submission.
GRAIL expects to complete the PMA modular submission for Galleri, which operates under Breakthrough Device Designation, in the first half of 2026.
PATHFINDER 2 represents the largest interventional study of a multi-cancer early detection test in the US to date, including adults aged 50 and older with no clinical suspicion of cancer, according to a release from GRAIL. The study’s primary objectives focus on evaluating safety and performance metrics including positive predictive value, negative predictive value, sensitivity, specificity, and cancer signal origin prediction accuracy.
Photo caption: Percent of cancers identified in PATHFINDER 2
Photo credit: GRAIL