Summary:
A new study from The First Affiliated Hospital of Zhengzhou University introduces a dynamic biomarker system and simplified genetic classifier to improve early risk detection and personalized treatment in differentiated thyroid carcinoma (DTC).
Takeaways:
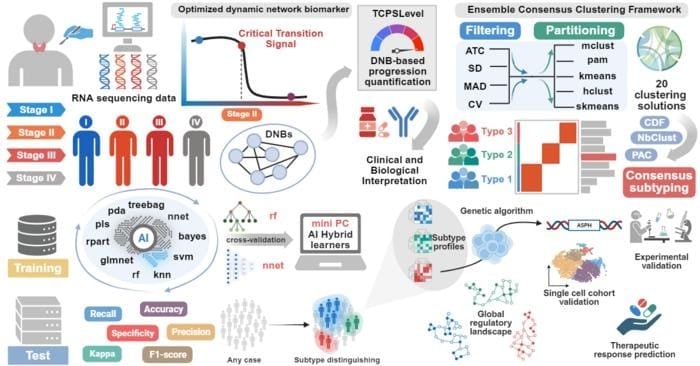
- Researchers identified Stage II as a critical tipping point in thyroid cancer progression using a dynamic network biomarker algorithm.
- The TCPSLevel score quantifies early molecular risk signals and outperforms traditional staging in predicting aggressive disease.
- A 12-gene classifier (miniPC) enables accurate molecular subtype prediction, supporting more precise and practical treatment strategies.
Differentiated thyroid carcinoma (DTC) is typically indolent, and some patients may be managed conservatively without immediate surgery. However, it remains a clinical challenge to determine who is suitable for active surveillance and to identify when thyroid cancer disease progression may occur.
A new study led by researchers at The First Affiliated Hospital of Zhengzhou University addresses this issue by developing an innovative dynamic biomarker system. Using an optimized dynamic network biomarker (DNB) algorithm, the team identified a “tipping point” in Stage II of thyroid cancer, where the disease shifts from a stable state to rapid progression. “Our analysis revealed that Stage II is a critical transition stage,” says corresponding author Prof. Xinguang Qiu.
To quantify individual risk, the team created TCPSLevel, a scoring system that captures early-warning molecular signals. Patients with high TCPSLevel had more advanced thyroid cancer disease and worse outcomes. “This score outperforms traditional staging in identifying high-risk individuals,” notes co-author Dr. Ge Zhang.
The researchers applied AI-based consensus clustering to over 1,100 thyroid cancer samples and identified three reproducible molecular subtypes, each with distinct immune profiles and progression risks. The most aggressive subtype was associated with the gene ASPH, which was experimentally validated.
To support clinical use, they developed a simplified classifier (miniPC) based on just 12 genes, enabling accurate subtype prediction across multiple datasets. “This tool offers a practical approach to personalized treatment planning,” says Dr. Haonan Zhang.
By integrating multi-omics data, machine learning, and single-cell analysis, the study provides new insights and tools for early risk stratification and targeted management of thyroid cancer.
Featured Image: How the ai-powered framework identifies critical transitions and classifies thyroid cancer subtypes. Image: The Authors