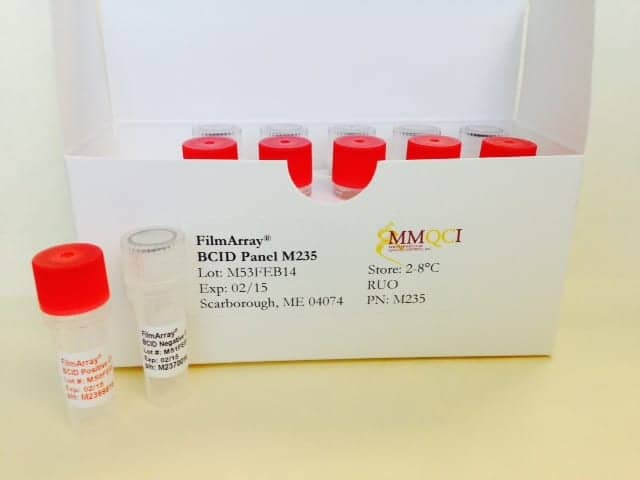
The FilmArray Blood Culture Identification Panel M235 from Maine Molecular Quality Controls, Scarborough, Me, is intended for research use only as a quality control to monitor the detection and identification of 24 pathogens and 3 antibiotic resistant genes associated with bloodstream infections when tested by the FilmArray instrument from BioFire Diagnostics. Two controls provide positive and negative results for each of the 27 targets. The panel comprises 12 tubes, 100µL each, of synthetic DNA suspended in a non-infectious solution of buffers, preservatives, and stabilizers. The panel is stable through the expiration date provided on the label when stored at 2° to 8°C and does not contain any biological material of human origin. For more information, visit Maine Molecular Quality Controls.
BCID Panel Provides Results for 27 Targets