CDC Selects Cepheid as National Collaborator for Rapid Diagnostic Development
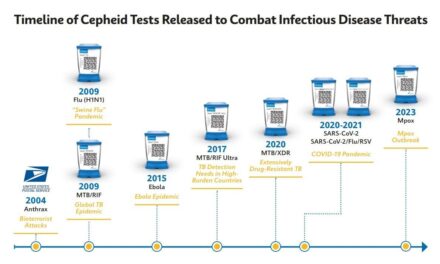
The CDC has named Cepheid one of four national collaborators to accelerate rapid diagnostic assay development for public health emergencies under a new federal initiative and IDIQ contract.