SeptiCyte RAPID demonstrated superior performance compared to procalcitonin and C-reactive protein in detecting sepsis among critically ill patients with hospital-acquired infections.
A molecular diagnostic test for sepsis showed significantly better diagnostic accuracy than traditional inflammatory biomarkers in critically ill intensive care unit patients with nosocomial infections, according to a study published in the journal Infection.
The prospective, observational study evaluated Immunexpress’ SeptiCyte RAPID’s ability to discriminate between sepsis and systemic inflammatory response syndrome (SIRS) compared to procalcitonin and C-reactive protein. The study enrolled 69 patients with ICU-acquired infection, which was confirmed in 50.7% of patients.
SeptiCyte RAPID achieved an area under the curve (AUC) of 0.995 (95% CI 0.930–1.003), substantially outperforming procalcitonin with an AUC of 0.600 (95% CI 0.454–0.745) and C-reactive protein with an AUC of 0.703 (95% CI 0.564–0.839). The test yielded a sensitivity of 91.4%, specificity of 73.5%, and positive and negative predictive values of 78% and 89.3%, respectively.
“In our study, we evaluated SeptiCyte RAPID for the identification of nosocomial sepsis, which is often difficult to diagnose due to overlapping inflammation from initial pathology, invasive devices, and noninfectious complications,” says Paula Ramírez Galleymore, MD, Hospital Universitario y Politécnico de la Fe and former president of the Scientific Committee of the Spanish Society of Intensive Care, Critical Care and Coronary Care Units, in a release. “Gene host response assessment proved effective in detecting sepsis in critically ill patients with nosocomial infections. Notably, 67.6% of patients without confirmed infections were initially treated with antimicrobials, suggesting SeptiCyte RAPID could help reduce unnecessary antimicrobial use.”
Potential for Antibiotic Stewardship
The study findings suggest the test could support antimicrobial stewardship efforts. Researchers noted that 67.6% of patients without confirmed infections were initially treated with antimicrobials, indicating potential for reducing unnecessary antibiotic use.
“Given its rapid turnaround time and objective output of SeptiScore, SeptiCyte RAPID may support antibiotic stewardship by guiding decisions regarding the initiation, escalation, or withholding of antimicrobials,” says Roy Davis, MD, PhD, MHA, chief medical officer of Immunexpress, in a release.
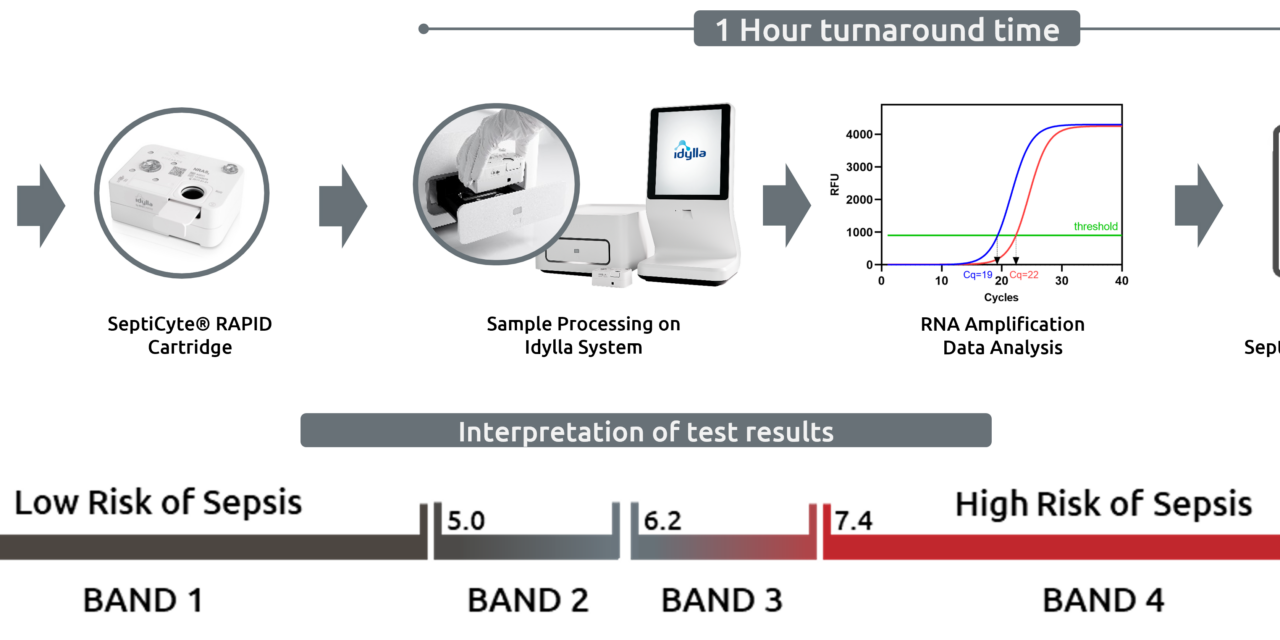
SeptiCyte RAPID is a sample-to-answer, cartridge-based molecular test that uses reverse transcription polymerase chain reaction to quantify host response gene expression from whole blood. The test generates a SeptiScore that falls within four discrete interpretation bands based on the increasing likelihood of sepsis.
The test runs on the Biocartis Idylla Platform and is intended for use in conjunction with clinical assessments, vital signs, and laboratory findings as an aid to differentiate infection-positive sepsis from infection-negative systemic inflammation response syndromes in patients with escalating signs and symptoms of critical illness.
SeptiCyte RAPID is CE marked for use in European Union member countries and those harmonized with the EU IVD Directive. The test received FDA clearance in November 2021 for use in hospitalized patients suspected of sepsis.
Photo caption: SeptiCyte RAPID workflow illustration
File photo/Immunexpress