New TheraPEAK products include mammalian-derived cytokines for immune cell expansion and chemically defined medium for AAV production.
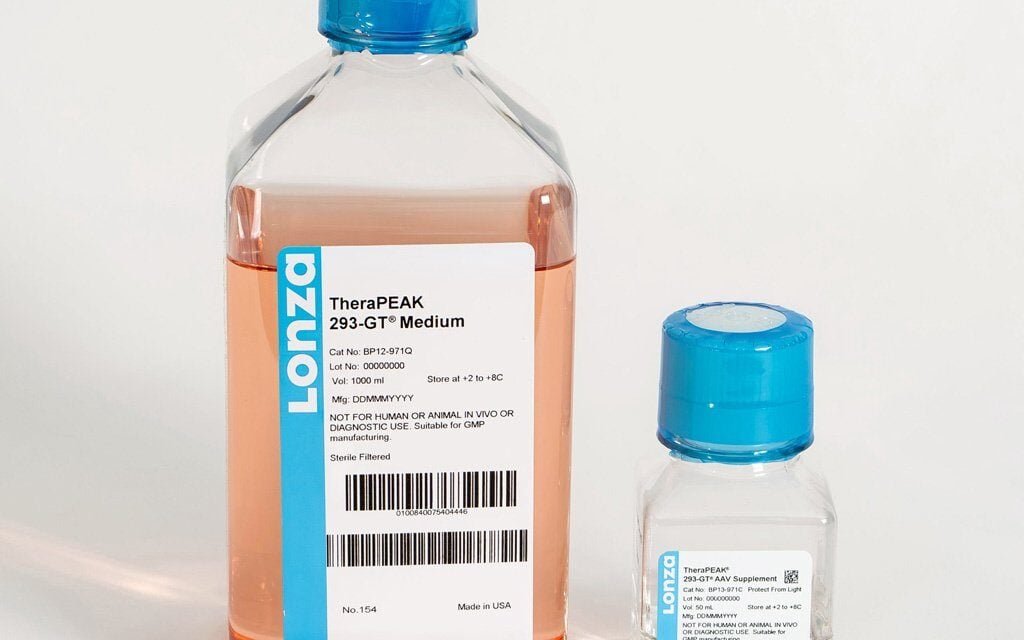
Lonza has launched TheraPEAK AmpliCell Cytokines and TheraPEAK 293-GT Medium to expand its GMP solutions for cell and gene therapy manufacturing.
The AmpliCell Cytokines are designed to support the expansion, activation, and differentiation of immune cells in cell therapy manufacturing. Produced in a mammalian expression system, the cytokines feature proper folding and glycosylation to deliver native-like structure and function, according to the company.
The mammalian production system provides superior biological relevance, batch-to-batch consistency, and greater predictability compared to bacterial systems in both research and GMP applications, Lonza says.
The 293-GT Medium is a chemically defined, animal-origin-free system optimized for adeno-associated virus production in suspension HEK293 cells. The medium provides a scalable option for gene therapy programs and is engineered to integrate with existing workflows.
The 293-GT System, which includes both growth medium and supplement, is compatible with commercially available transfection reagents and AAV enhancers. The system delivers strong AAV titers and supports high full-to-empty capsid ratios, according to Lonza.
Clinical Trial History
TheraPEAK products have been used in FDA-approved therapies and more than 130 clinical trials globally. The newly added solutions provide researchers and manufacturers with performance-focused, scalable, and regulatory-ready options that streamline cell and gene therapy workflows from discovery through clinical development.
“The introduction of AmpliCell Cytokines and the 293-GT Medium to our TheraPEAK Range greatly enhances our offering for cell and gene therapy customers, providing them with reliable solutions to support their drug development efforts,” says Mike Goetter, head of bioscience, specialized modalities, Lonza, in a release.
The products address critical components in cell and gene therapy manufacturing, where cytokines support the expansion and maintenance of living cells, while the viral production medium enables AAV vector manufacturing for gene therapy applications.