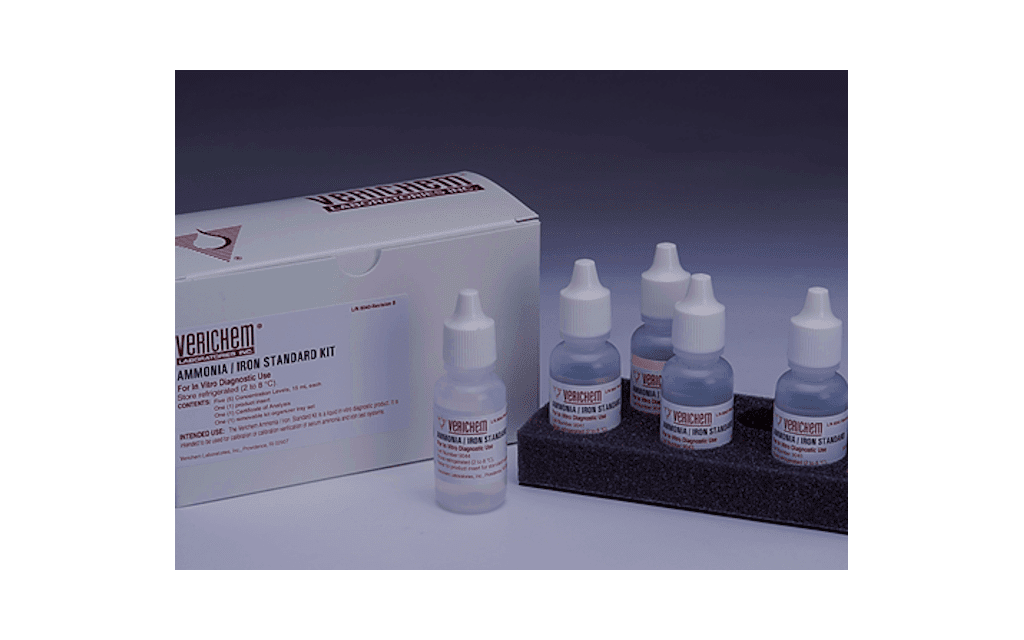
Verichem Laboratories announced the open vial stability claim on its combined Ammonia/Iron Standard Kit has extended to 24 months when stored at 2°C to 8°C. As a result, this liquid-stable, ready-to-use product is ideal for numerous calibration verification events or test system troubleshooting.
Verichem’s reference standards are explicitly designed and manufactured to meet the needs of a variety of laboratory professionals involved in routine clinical testing, research applications, or in vitro diagnostic (IVD) manufacturing facilities. These unique reference standards are treated exactly as patient specimens with a variety of clinical testing systems, including those from Abbott Diagnostics, Beckman Coulter, Medica Corporation, Roche Diagnostics, and Siemens Healthineers, among many others.
The five-level Ammonia/Iron Standard Kit, along with an optional and standalone sixth level, comprise this set of definitive standards containing concentration levels of iron from 10 mcg/dL to 1000 mcg/dL and 10 mcg/dL to 2000 mcg/dL for ammonia. The product is intended for the calibration or calibration verification of both ammonia and iron assays employing either enzymatic or colorimetric wet chemistry testing methodologies.
The liquid-stable format, which is free of glycols, surfactants, azides, and other interfering substances, and the aqueous matrix is uniquely designed to support these critical trace analytes, while offering exceptional lot-to-lot duplication and product performance claims. The set point design, with equidistant target concentrations, addresses the current CLIA requirements for calibration verification materials used for the determination of a clinical system’s accuracy, sensitivity, linearity, and reportable range.
All kit materials are filled inside convenient, easy-to-use, 15 mL translucent, polyethylene dropper vials, and prepared from reagent-grade source materials. Each kit is certified using available standard reference materials (SRM) from the National Institute of Standards and Technology (NIST), while ammonia concentrations are certified using American Chemical Society (ACS) reagent-grade independent primary standards. In addition, a certificate of analysis (COA) indicating traceability and actual lot data is provided with each kit.
For additional information, visit Verichem Laboratories.
Featured image: Ammonia/Iron clinical reference standard kit (Courtesy: Verichem Laboratories)