The AACC is urging clinical lab professionals to write to their legislators to keep the VALID Act out of a large Fiscal Year 2023 “omnibus” appropriations bill, thus giving Congress and stakeholders an opportunity to work out an acceptable solution that will serve all parties, particularly patients.
The association wants professionals in the industry to quell the misconception that the bill has addressed earlier concerns regarding the VALID Act and continue to impress upon legislators the negative impact it will have on clinical laboratories
In a public outreach to clinical lab professionals, the AACC said: “Supporters of VALID are seeking to include the measure in the must-pass FY23 budget agreement, which is nearing completion in Congress. Backers of VALID seek to claim the bill now addresses the lab community’s concerns, pointing to new language that has not been vetted and introduces more problems than solutions. AACC needs your continued help to keep this from happening.”
The new version of the bill is claimed to exempt academic medical centers from the new regulatory framework in FDA. However, upon close inspection, the exemption proposal is too narrow, making it meaningless, the AACC says.
“For example: if the physical laboratory is in a different building from the emergency room or the hospital where the patient is being treated—which is often the case—the test is not exempt,” that AACC says. “If another FDA-approved test is already on the market, a test is not exempt. Similarly, if there was no test for a condition, and a lab developed an LDT, as soon as the FDA cleared or approved another test kit the lab would need to stop performing the LDT or seek FDA review.
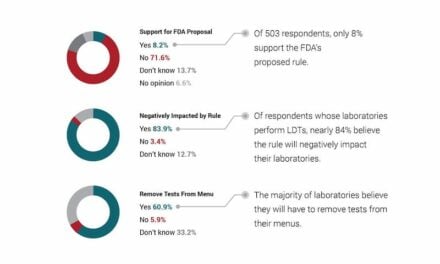
“The other duplicative regulatory and inspection requirements, as well as user fees, remain in the bill, leaving laboratories exposed to significant new fees and regulatory burden. None of provisions in this bill will improve patient care. Instead, labs will be forced to curtail the number and types of LDTs they perform, limiting patient access to essential testing and potentially delaying care.”
As the 117th Congress wraps up and supporters of VALID ramp up, AACC requests clinical lab professionals continue to contact their legislators.