For laboratories seeking to make the shift to an individualized quality control plan under the guidelines implemented by the Centers for Medicare and Medicaid Services (CMS), getting up to speed on IQCP requirements has to be the first step. But gaining access to the right assortment of tools will also be worth some early thought, especially as a lab’s tool selections are likely to be as individualized as their new approach to quality control. Below are profiles of half a dozen companies and product lines that may contribute to lab planning on the way to implementing an IQCP.
Audit MicroControls
The company’s Control FD Assayed Chemistry is a reference control consisting of human serum-based solutions. It is intended to simulate human serum samples in order to monitor the precision of laboratory testing procedures for a number of general chemistry assays. This lyophilized bilevel reference control has a shelf life of 1 year and an open-vial stability of 14 days, and is packaged as 10 5-mL vials. Each of the two levels is sold individually; both have been approved for use with
22 different analyzer models.
Clinical Software Solutions
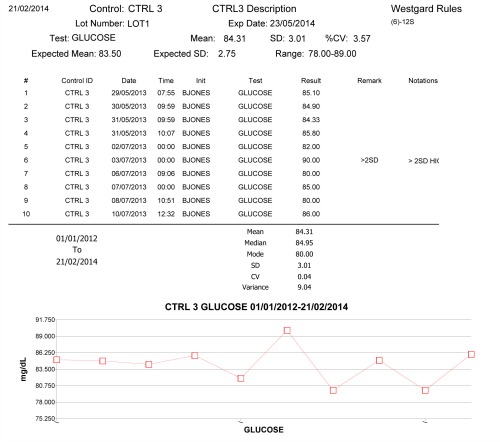
The company also offers tools to support quality assurance and document scanning. These tools support a facility’s implementation of IQCP by providing tracking for external documents such as control package inserts, policies and procedures, and third-party documentation, as well as tracking of processes and tasks such as multiple checklists, accreditation, staff training, and so on.
Randox Laboratories
Data management will play a vital role in fulfilling the risk-assessment requirements described in CMS’s IQCP guidelines. “Labs can make use of historical data they already have, such as data from proficiency testing,” says Steven Jordan, global business manager for Randox Quality Control, Crumlin, United Kingdom. “They can use data from the verification of their test systems as well as manufacturer information.”
However, the use of external data must be complemented by a laboratory’s own in-house data, as established by its own personnel in its own unique environment, says Jordan. “This data must demonstrate the stability of the test system and support the number and frequency of quality control activities documented in the quality control plan.”
Interlaboratory data-management systems, such as Randox’s Acusera 24-7 platform, can help laboratories ensure that errors are not just identified but appropriately documented, monitored, and acted upon to prevent future errors. Acusera 24-7 offers an easy-to-use interface, fully interactive charts, and detailed reports that support analysis of lab performance. In addition, says Jordan, the system provides access to “a large pool of peer-group data that’s updated at least every 24 hours, allowing comparison of data from multiple instruments and laboratories across the globe.”
Radiometer America

However, some manufacturers view even the IQCP risk-mitigation requirements as a minimum standard, and additionally follow guidelines of the International Organization for Standardization (ISO)—specifically, the standard for risk management in medical devices, ISO 14971—which requires manufacturers to identify the hazards associated with their devices, estimate and evaluate the associated risks, control the risks, and monitor the effectiveness of their controls. All Radiometer blood gas analyzers have been developed to be compliant with ISO 14971.
“Since risk mitigation is part of our analyzers’ ‘design DNA,’ we now have the base of information that laboratories will need in order to develop their specific IQCP plan as it relates to our analyzers,” says Sy Albakri, PhD, manager for clinical and scientific affairs at Radiometer America, Westlake, Ohio. “It is our goal, during the educational period, to offer our customers this information in a form that will assist them in creating their IQCP plans.”
Thermo Fisher Life Sciences Solutions
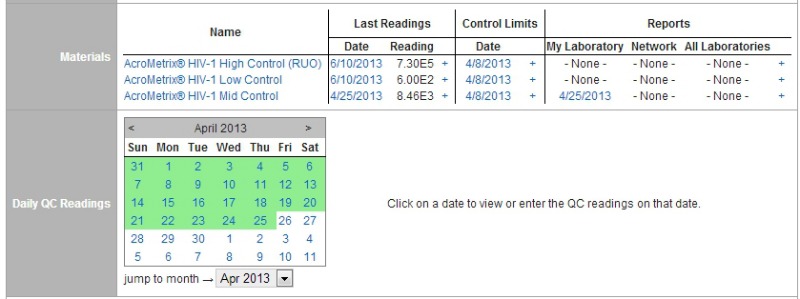
AcroMetrix molecular diagnostic controls are produced in a manufacturing site compliant with FDA current good manufacturing practices (cGMPs) and certified to the ISO standard for quality systems in medical devices, ISO 13485. Manufacturing of the controls also adheres to the ISO standard for the metrological traceability of calibrators and control materials, ISO 17511, ensuring material consistency from lot to lot. The controls are manufactured to mimic patient-sample behavior as closely as possible.
AcroMetrix and its products were acquired in 2010 by Life Technologies, which has in turn recently been acquired by Thermo Fisher Scientific. In February 2014, Thermo Fisher announced that it would join its previous biosciences business with units of Life Technologies to form a new business segment called Thermo Fisher Life Sciences Solutions.
Meanwhile, AcroMetrix-branded software solutions are also helping labs to design validation studies and perform the statistical analyses needed to comply with IQCP requirements. The EZQC Online tool enables users to design verification and validation studies based on the ISO standard for quality management in medical laboratories, ISO 15189, or on guidelines issued by New York State, by the College of American Pathologists, or by the Centers for Medicare and Medicaid Services for compliance with requirements of the Clinical Laboratory Improvement Amendments of 1988 (CLIA). The software also facilitates tracking and carrying out antimicrobial resistance assessments, and performing lot-to-lot QC monitoring.
“EZQC Online allows labs to consolidate and manage data to easily put together reports for IQCP compliance, regardless of the complexity of a laboratory’s specific IQCP protocol,” says Alan Neo, product manager for Thermo Fisher Life Sciences Solutions, in Benicia, Calif.
Viewics
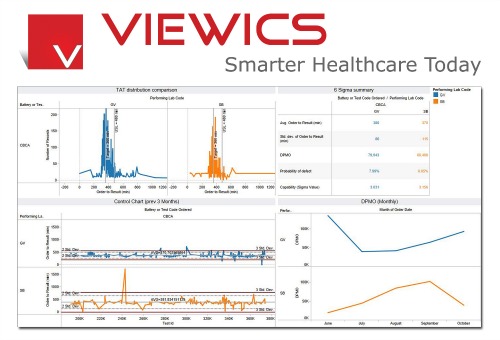
“VHI has changed the way laboratories think about the use of their data,” says Tim Kuruvilla, vice president for sales and marketing at Viewics Inc, Sunnyvale, Calif. “By creating analytics solutions that are truly intuitive and easy for clinical and quality staff to use, Viewics has created a paradigm shift.”
Historically, says Kuruvilla, there has been a significant gap between those who needed information (laboratory professionals) and those who could actually extract the data (IT). “By empowering laboratorians with information at their fingertips,” he says, “laboratories can truly have a culture of continuous quality improvement.”