Summary: Researchers have developed a highly accurate urine-based diagnostic kit for bladder cancer, offering a convenient, non-invasive solution for early detection and continuous monitoring at home.
Takeaways:
- High Accuracy and Early Detection: The kit demonstrated an 88.8% sensitivity in clinical trials, significantly outperforming existing commercial tests, and effectively detects early-stage bladder cancer.
- Non-Invasive and User-Friendly: Utilizing a unique water-oil layering mechanism, the kit eliminates the need for painful cystoscopies, enabling precise biomarker detection without urine sample preprocessing.
- Commercialization and Future Applications: The research team is establishing a startup, FloatBioscience, to mass-produce and commercialize the diagnostic kit, with plans to expand its use for other diseases.
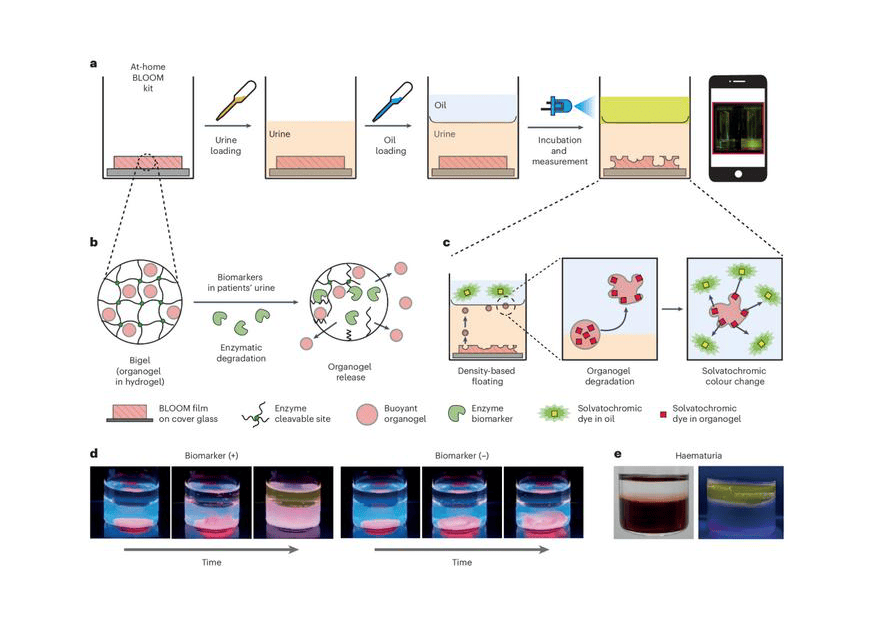
A research team from the Korea Institute of Science and Technology has developed a urine-based diagnostic kit for bladder cancer that can be conveniently used at home. This kit can accurately detect bladder cancer biomarkers without any preprocessing of urine samples.
Impact of Bladder Cancer
Bladder cancer has a cure rate of over 90% when detected early, but it has a high recurrence rate of 70%, necessitating continuous monitoring. Late detection often requires major surgeries such as bladder removal followed by artificial bladder implantation or the use of a urine pouch, significantly lowering the patient’s quality of life.
However, existing urine test kits have low sensitivity, and cystoscopy, which involves inserting a catheter into the urethra for internal bladder examination, is both painful and burdensome. This highlights the urgent need for a simple yet accurate diagnostic technology for patients.
Developing the Bladder Cancer Home-Detection Kit
The research team, led by Youngdo Jeong, PhD, of the Center for Advanced Biomolecular Recognition the Korea Institute of Science and Technology (KIST, President Oh Sang-Rok), in collaboration with Professor Seok-Ho Kang’s team from the Department of Urology, Korea University College of Medicine, designed the diagnostic kit by utilizing the principle of water and oil layering to detect bladder cancer biomarkers.
Biomarker detection in urine has been challenging due to the low concentration of biomarkers and interference from impurities like hematuria. The newly developed kit overcomes these issues by employing a mechanism where a film bonded to the biomarker is broken, releasing a buoyant signal carrier that moves to the oil layer and emits a detectable signal. This design prevents interference from impurities like hematuria and amplifies the signal, enabling precise biomarker detection.
Clinical Trial of the Bladder Cancer Test
In clinical trials conducted with 80 patients and 25 healthy individuals at Korea University’s Department of Urology using a double-blind methodology, the diagnostic kit achieved a sensitivity of 88.8%. This is a significant improvement compared to the mere 20% sensitivity of existing commercial tests. Notably, while conventional methods are almost incapable of diagnosing early-stage bladder cancer, the new kit accurately detects even early-stage cases.
This diagnostic kit offers a revolutionary approach for the early detection of bladder cancer through non-invasive and simple urine tests. It is expected to reduce the need for unnecessary cystoscopy, improve survival rates through early detection, and enhance patients’ quality of life. Building on these results, the research team aims to develop products for mass and rapid use in comprehensive medical examination centers and easy application at home.
“This study demonstrates the potential for early bladder cancer diagnosis using a simple diagnostic kit, reducing the need for unnecessary cystoscopies,” says Jeong.
Professor Seok-Ho Kang from Korea University added, “This research, a result of translational studies between KIST and Korea University, paves the way for early diagnostic technologies for various diseases beyond bladder cancer.”
Notably, Youngdo Jeong and Professor Seok Ho Kang, in collaboration with Dr. Dong Jin Lee of the Korea Institute of Machinery & Materials, plan to establish a startup, “FloatBioscience,” focusing on the commercialization of this bladder cancer diagnostic kit, which allows for uniform mass production. The startup has been selected as a preliminary convergence startup team for the 2024 NST Convergence Startup Challenge.
Featured image: a, Schematic illustration of the workflow of the BLOOM assay. b,c, Mechanism of organogel messenger release from the biomarker-degradable bigel film (b) and solvatochromic dye transfer to the organic layer via density-based floating (c). d,e, Photograph of biomarker detection using the BLOOM assay in the biphasic system under UV light irradiation in urine (d) and haematuria (e) samples. Photo: Korea Institute of Science and Technology