FibroSIGHT Plus introduces automated quantitative analysis using stain-free imaging technology to improve consistency in liver biopsy evaluation.
HistoIndex has launched FibroSIGHT Plus, its second laboratory-developed test available in the US market, introducing AI-based quantitative analysis for fibrosis assessment in patients with metabolic dysfunction-associated steatohepatitis (MASH).
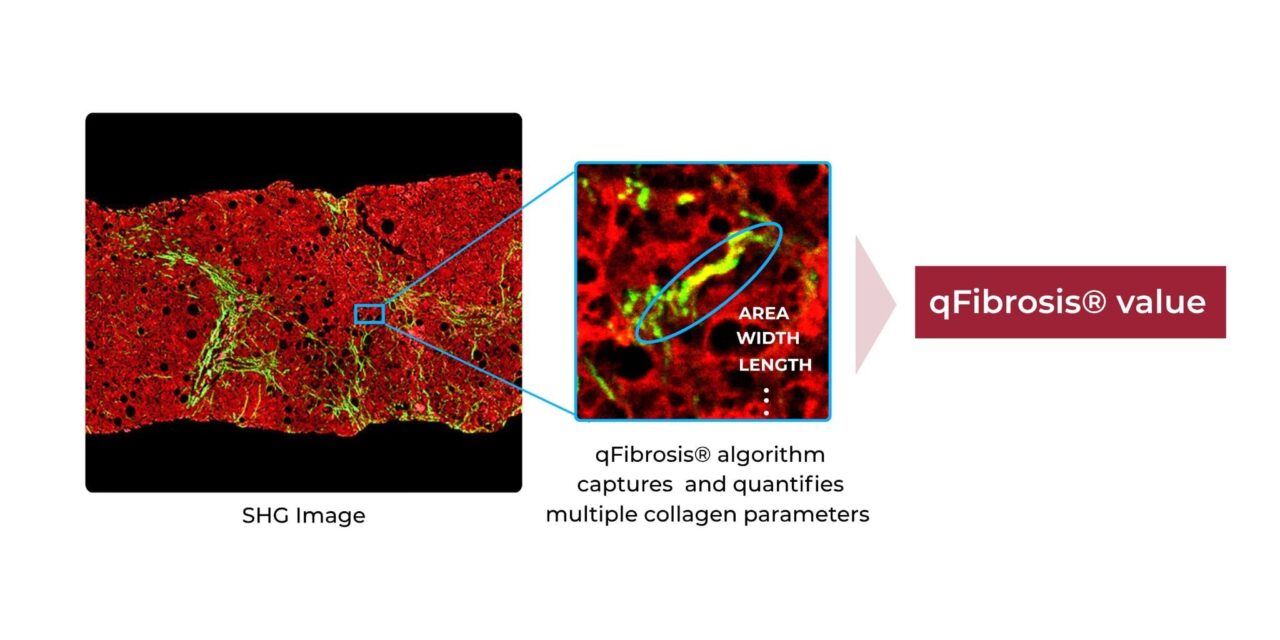
The new test builds on the company’s original FibroSIGHT platform by adding qFibrosis, HistoIndex’s proprietary AI-driven algorithm that uses stain-free second harmonic generation (SHG) imaging to automatically quantify multiple fibrosis-related collagen features in liver biopsies.
“We’re thrilled to deliver another advancement in MASH diagnostics, bridging our experience and capabilities in clinical trials all the way to clinical use,” says Yukti Choudhury, chief development officer at HistoIndex, in a release.
Continuous Scale Measurement
The qFibrosis algorithm analyzes collagen architectural and morphological features detected by SHG imaging across various spatial zones of liver biopsies, translating them into a single value or stage for fibrosis. This approach provides fibrosis measurement on a continuous scale rather than discrete categories, potentially offering more detailed insights into disease severity.
The technology aims to address variability issues associated with conventional pathology methods, which can be affected by inconsistencies in staining and interpretation. FibroSIGHT Plus delivers standardized measurements across entire biopsy specimens, removing subjectivity and inter-reader variability.
“Having worked with HistoIndex for many years, I have witnessed firsthand the value that Second Harmonic Generation imaging brings to fibrosis assessment in MASH clinical trials, with highly sensitive and consistent detection and quantification of fibrosis in liver biopsies,” says Naim Alkhouri, MD, chief academic officer at Summit Clinical Research and director of the Steatotic Liver Disease Program at the Clinical Research Institute of Ohio, in a release.
Clinical Validation Data
According to HistoIndex, when benchmarked against reference fibrosis stages from expert pathologists’ consensus, qFibrosis aligns closely and demonstrates greater consistency than traditional histology methods.
“When benchmarked against reference fibrosis stages from expert pathologists’ consensus, HistoIndex’s qFibrosis aligns closely, and demonstrates greater consistency than traditional histology. This level of reproducibility is essential in clinical practice and represents an important step toward replacing subjective scoring with reliable, quantitative metrics,” says Mazen Noureddin, MD, professor of medicine and transplant hepatologist at Houston Methodist Hospital and co-chairman of the board at Summit and Pinnacle Clinical Research, in a release.
The launch addresses growing demand for more accurate assessment tools in MASH diagnosis, as pathologist assessment of liver biopsy remains the gold standard for diagnosing and assessing disease severity. Traditional histological scoring systems used as surrogate endpoints in clinical trials are limited in capturing the complex nature of the disease.
MASH is a progressive form of metabolic dysfunction-associated steatotic liver disease characterized by steatosis, ballooning degeneration, and inflammation, which can lead to fibrosis, cirrhosis, liver failure, and increased risk of liver cancer.
Photo caption: Quantifying multiple collagen parameters with qFibrosis
Photo credit: HistoIndex
We Recommend for You: