Scientists of the National Institutes of Health (NIH) have developed an ultrasensitive new test to detect abnormal forms of the protein tau associated with uncommon types of neurodegenerative diseases called tauopathies. This advance raises hope of using cerebrospinal fluid (CSF)—an accessible patient sample—to diagnose these and possibly other more-common neurological diseases, such as Alzheimer’s disease. The new test is called 4R RT-QuIC—which stands for four-repeat tau protein amplified in a real-time, quaking-induced conversion process.1
Scientists have linked the abnormal deposition of tau in the brain to at least 25 different neurodegenerative diseases. However, the only way to accurately diagnose these diseases has been to analyze brain tissue after the patient has died.

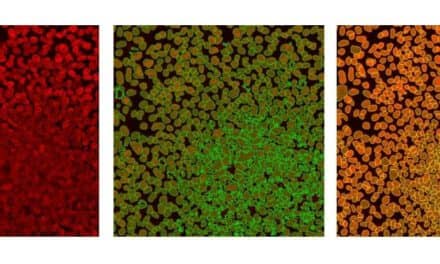
Representative negative-stained transmission electron microscopy images of 4R RT-QuIC products seeded with brain homogenates from individuals with the designated diseases: corticobasal degeneration; frontotemporal dementia and Parkinsonism linked to chromosome 17; and progressive supranuclear palsy. Photo courtesy National Institute of Allergy and Infectious Diseases (NIAID).
For their study, the NIH researchers began with the same test concept they developed when using postmortem brain tissue samples to detect the abnormal tau types associated with Alzheimer’s disease, chronic traumatic encephalopathy (CTE), and Pick disease. They then adapted the test to use CSF samples to look for the abnormal tau of progressive corticobasal degeneration (CBD), supranuclear palsy (PSP), and other less-common tauopathies.
They detected abnormal tau in CSF from both living and deceased patients. In one case, the test led to a corrected diagnosis in a patient who had died from CBD but who was initially diagnosed with PSP.
The test was developed at NIH’s Rocky Mountain Laboratories, part of the National Institute of Allergy and Infectious Diseases. Study collaborators who provided patient specimens and clinical guidance included investigators from the Mayo Clinic in Jacksonville, Fla; Indiana University; the University of Bologna; the University of California, San Diego; the University of California, San Francisco; and the University of Verona.
The researchers plan to continue evaluating the clinical performance of 4R RT-QuIC by analyzing larger sets of CSF samples. One focus will be to compare test results from tauopathy patients who agree to provide CSF samples both before and after death. The scientists hope this type of evaluation will help them better understand how abnormal tau in CSF evolves during brain disease.
For further information, visit the National Institute of Allergy and Infectious Diseases (NIAID).
Reference
- Saijo E, Metrick MA II, Koga S,et al. 4-repeat tau seeds and templating subtypes as brain and CSF biomarkers of frontotemporal lobar degeneration. Acta Neuropathol. Epub ahead of print, October 16, 2019; doi:10.1007/s00401-019-02080-2.
Featured image:
Detail of representative negative-stained transmission electron microscopy images of 4R RT-QuIC products seeded with brain homogenates from individuals with uncommon types of neurodegenerative diseases called tauopathies. Photo courtesy National Institute of Allergy and Infectious Diseases (NIAID).