Developed in the 1950s and 1960s, dried blood spot testing provides clinical labs an easy-to-collect, reliable, and cost-effective means to test for a variety of diseases.
By Susan Zneimer, PhD, FACMGG; Donna Hongo, PhD, CLS; Maite Sabalza, PhD; and Ilana Heckler, PhD
Dried blood spots (DBS) refer to a whole blood sampling technique that requires only a small volume of patient specimen. Blood is collected through a finger prick and spotted on a special filter paper, dried, and then sent to the lab for analysis.
DBS were developed after the Second World War.1 The first studies on DBS were published in the 1950s for detection of syphilis infection.2 Robert Guthrie introduced DBS in clinical testing in the 1960s for neonatal screening of phenylketonuria, an inherited metabolic disorder. Since then, DBS have been used as an alternative to serum or plasma for newborn screening of phenylketonuria and other disorders. Specifically, DBS from a heel prick are collected in over 50 million newborns annually.3 In addition to newborn screening, DBS have been used for detection of infectious diseases in the general population, such as antibodies against HIV, Epstein-Barr virus and HCV.4 DBS can be also used as a sample for drug development, monitoring therapeutics, toxicokinetic, and pharmacokinetic studies, among other applications.1
Advantages of Using DBS
In clinical testing, collection of DBS is a cost-effective alternative to venipuncture.5,6 Additionally, it is non-invasive, easy to collect, and reduces the risk of bacterial contamination or hemolysis.7 Since DBS are convenient for patient sampling, it can be self-collected at home by patients without the assistance of medically trained personnel. DBS also have a high stability during storage and shipping which makes it feasible to collect blood samples in large populations.6,8 Furthermore, a variety of adverse complications may be encountered during blood collection process.9 In the current world direct-to-consumer (DTC) testing, where patients can directly order their preferred clinical tests online, the need for an in-person doctor visit can be avoided.
Technical Considerations for Dried Blood Spot Testing
DBS Card
Two types of filter paper are suitable to be used in the card for DBS collection: pure cotton filter paper and glass microfiber paper. Depending on the analyte to be detected, one type is preferred over the other. This is because the adsorption and dispersion of the DBS are affected by thickness and density of the filter paper.10 Dried blood spot testing cards are commercially available.10
Sample Collection
It is important to thoroughly clean your hands before sample collection to avoid contamination. A lancet is used to draw a drop of whole blood onto the card into a specific target area (circle) printed on the paper (Figure 1 (a)). The whole blood drop should be spotted onto the center of the circle to ensure radial dispersion of blood to the edges so that enough volume of blood is collected (Figures 1 (b-c)). While the edges typically contain less amount of the analyte, the center of the spot contains the maximum concentration of the analyte. The hemoglobin and hematocrit sample level also affect the radial dispersion of the blood.11 It is also critical not to push onto the center of target area when spotting the blood. Instead, the drop of blood should fall onto the card as a result of gravity.
Proper sample collection technique is critical for an accurate analysis. Therefore, appropriate collection instructions must be distributed together with the DBS cards and the collection kit. Instructions should include detailed information regarding the cleaning procedure before sample collection, the best place for the finger prick, prick depth and the pressure to drop the blood. Instructions should also provide guidance on where and how to spot the blood and the time required to dry the samples.
a)

b)

c)

Extraction Protocol
Spot punches
Once the DBS are dried, the next step is to extract the analyte for clinical testing. Only a segment or hole punch is required for analysis and not the entire blood-filled circle (Figure 2). This can be performed with a manual or automated puncher. It is recommended to take the DBS punch from the center, or close to the edge, due to the high analyte concentration in these areas. The punch size may vary from 3 to 6 mm depending on the analyte tested or the clinical assay. As hematocrit can affect the performance of the clinical testing, some alternative techniques are available, such as blotting lower volume of whole blood on a smaller pre-cut filter paper and using the entire disk for analysis.11
Elution
Since the sample volume collected with DBS is minimum, the extraction and elution protocol need to be very accurate to have a high recovery rate. Different analytes can be detected using DBS including antibodies, hormones, and antigens from pathogens. These are different molecules that might diffuse at different rates through the filter paper with a high or low efficiency. Therefore, the elution steps need to be optimized based on the analyte that is detected.
High-quality Assays for Analyte Measurement
Once the analyte is extracted from the DBS, the next step is to measure it. There are different commercially available assays that have been validated with DBS using different technologies, such as ELISA, immunoblots, and mass spectrometry.4,13
Dried Blood Spot Testing at Symbiotica
Current Testing
Vacaville, Calif.-based Symbiotica, Inc., a clinical laboratory that provides expert consulting services in quality and regulatory compliance, and laboratory services that include assay development, has validated DBS as a sample type for different serological assays in the field of allergy and autoimmune disease, and was among the first labs with Emergency Used Authorization (EUA) granted by FDA for COVID-19 serology.14 The lab tested more than a thousand patient samples self-collected on dried blood spot testing cards in 2021. Due to the current ongoing pandemic, testing services were focused on COVID-19 serology testing. However, not all samples were accepted for testing in 2021. The main reason was that some samples were rejected due to “quantity not sufficient” which accounted for less than 1% of the tested samples. This was attributed to patients having difficulty in collection or lancet malfunction. Patients were provided with new kits and asked to recollect when samples were rejected.
Dried Blood Spot Testing Validation Studies: COVID-19 Serology
Symbiotica’s test was among the first at-home collection testing DBS granted EUA by FDA for COVID-19 serology. Below we describe the main studies required for validation of DBS as a sample type for testing. Additional data and required studies can be found in the EUA submission.14 EUROIMMUN Anti-SARS-CoV-2 ELISA (IgG) is the serological assay used for DBS testing. The results are reported qualitatively (positive, borderline, or negative) based on a ratio value.
Lay-User study
At Symbiotica, we first performed a lay-user or usability study. Participants were specifically selected who have either never used a self-collection device before or never had any clinical or medical experience. The goal of the study was to assess the ease of use of the DBS card to self-collect their sample. A total of 52 study participants were recruited for this study. The collection was conducted at simulated environments using one of the participant’s homes over two different dates (“events”); 98% of participants successfully collected DBS. The participant that was unsuccessful in the self-collection process has a known condition that makes blood collection difficult but wanted to participate in the study. This outcome was not unexpected. Additionally, a questionnaire was also shared with all the participants and 92.5% of them responded with positive feedback on the self-collection process.
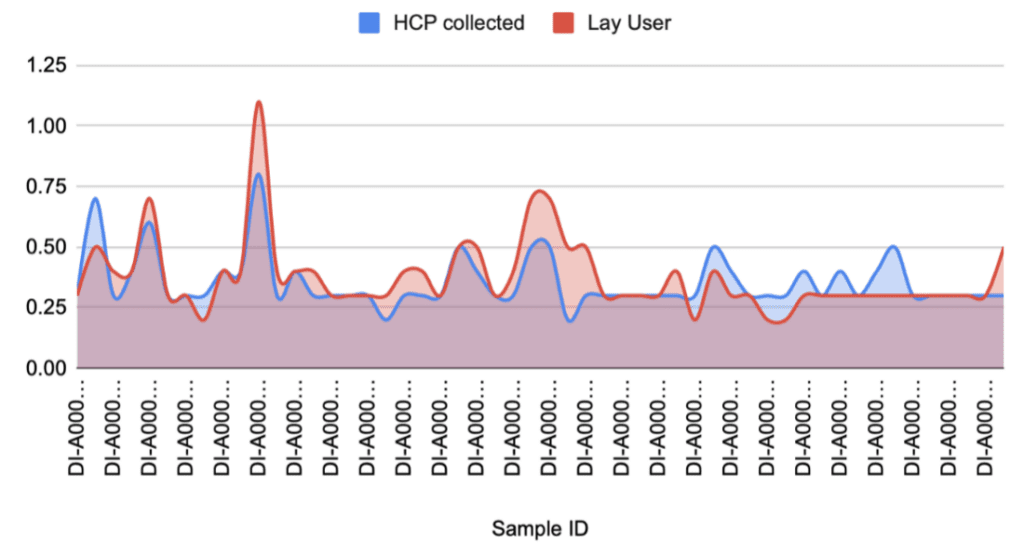
Correlation Study DBS and Serum
The correlation study was performed with 40 paired samples. The results showed 100% of concordance between DBS and serum.
Stability Studies
The DBS device stability was validated up to seven months per FDA EUA for EUROIMMUN Anti-SARS-CoV-2 ELISA (IgG). This was established using low positive samples applied to fresh cards and onto cards stored at room temperature for eight months. Results showed 100% of concordance between both cards.
DBS shipping stability was validated during a 192-hour (8-day) period at the customer’s house followed by a 144-hour (6-day) shipping cycle using both “winter” and “summer” shipping profiles.14 A total of 20 low-positive, 10 moderate/high-positive, and 10 negative contrived samples were prepared on three sets of DBS cards. One set of DBS cards was not exposed to any shipping profile conditions and served as the ‘baseline’ for the study. Set 2 of the DBS cards was exposed to the winter profile temperatures and set 3 was exposed to the summer profile temperatures. Symbiotica had successfully completed both the winter and summer shipping profiles for a study designed to replicate worst-case scenarios of a 192-hour (8-day) period at the customer’s house followed by a 144-hour (6-day) shipping cycle.14
Robustness Studies
Drying Time
Studies to assess the effect on the results of the time required to dry the DBS samples were performed. Different drying times were tested resulting in 100% positive percent agreement (PPA) and 100% negative percent agreement (NPA) with the recommended drying time (Table 1).
| IgG Positive results | IgG PPA | IgG Negative Results | IgG NPA | |
| Number of Samples Tested | 5 | 5 | ||
| 8 mins | 5 | 100% | 5 | 100% |
| 15 mins | 5 | 100% | 5 | 100% |
| 23 mins | 5 | 100% | 5 | 100% |
| 30 mins | (Recommended) | |||
| 45 mins | 5 | 100 (%) | 5 | 100% |
| 90 mins | 5 | 100 (%) | 5 | 100% |
| STUDY SUMMARY | 25 | 100% | 25 | 100% |
Specimen Volume (Number of DBS Punches)
Studies to assess the effect of the sample volume (number of DBS punches) were performed testing 2x below and 2x above the recommended volume. Results showed 100% PPA and 100% NPA with the recommended volume (Table 2).
| IgG Positive results | IgG PPA | IgG Negative Results | IgG NPA | |
| Number of Samples Tested | 5 | 5 | ||
| 1/2 spot | 5 | 100% | 5 | 100% |
| 1 spot | (Recommended) | |||
| 2 spots | 5 | 100% | 5 | 100% |
| STUDY SUMMARY | 10 | 100% | 10 | 100% |
Sample Diluent Volume
Studies to assess the effect of the sample diluent volume on the results were performed testing a diluent volume of 2x below and 2x above the recommended one. Results showed 80% PPA and 100% NPA (Table 3) with the recommended volume. The low PPA can be explained by an error only possible at lab processing. The risk of having this error is mitigated through proper training and competency, accurate SOPs, and process visuals at the lab bench.
| IgG Positive results | IgG PPA | IgG Negative Results | IgG NPA | |
| Number of Samples Tested | 5 | 5 | ||
| 125 µL sample diluent | 5 | 100% | 5 | 100% |
| 250 µL sample diluent | (Recommended) | |||
| 500 µL sample diluent | 4 | 80% | 5 | 100% |
| STUDY SUMMARY | 9 | 90% | 10 | 100% |
Temperature and Humidity
Studies to assess the effect of temperature and humidity conditions on the results were performed. Results showed 100% PPA and 100% NPA with the recommended conditions (Table 4).
| IgG Positive results | IgG PPA | IgG Negative Results | IgG NPA | |
| Number of Samples Tested | 5 | 5 | ||
| Overnight in Incubator (50°C) | 5 | 100% | 5 | 100% |
| Room Temperature Controlled | (Recommended) | |||
| Overnight in Refrigerator (4°C) | 5 | 100% | 5 | 100% |
| Overnight in Freezer (-20°C) | 5 | 100% | 5 | 100% |
| Overnight in water | 5 | 100% | 5 | 100% |
| STUDY SUMMARY | 20 | 100% | 20 | 100% |
Light
Studies to assess the effect of the light on the results were performed using different light conditions: fluorescent, incandescent, and natural lighting. Results showed 100% PPA and 100% NPA (Table 5).
| IgG Positive results | IgG PPA | IgG Negative Results | IgG NPA | |
| Number of Samples Tested | 5 | 5 | ||
| Fluorescent | 5 | 100% | 5 | 100% |
| Incandescent | 5 | 100% | 5 | 100% |
| Natural lighting | 5 | 100% | 5 | 100% |
| STUDY SUMMARY | 15 | 100% | 15 | 100% |
Future Perspectives
There is an increasing interest and demand for DBS testing to extend the reach of global health and disease surveillance programs to hard-to-reach populations. DBS offers a cost-effective solution for multiple use cases by simplifying logistics for collecting, preserving, and transporting blood specimens in settings with minimal infrastructure. In addition to this, DBS can be assisted- or self-collected.
Self-collection allows using DBS as a sample in DTC testing. These tests are available to people without the need for healthcare providers, can be ordered online or over the counter at the pharmacy depending on the country and state. The COVID-19 pandemic has brought testing very close to our lives, and as a result, the DTC testing market is growing. The general population is more familiar and interested in DTC testing. However, self-collection might be challenging for some patients. For this reason, it is critical to properly provide instructions for self-collecting the sample and for shipping it to the lab. Lay-user studies help to assess the instructions provided by the labs.
Regardless of the clinical applications of DBS testing, there are some non-technical barriers to implement it. For example, only very few DBS tests are cleared/approved by FDA. Laboratories need to validate DBS testing with the corresponding assay as a lab-developed test (LDT) based on state and local guidance. DBS are a different matrix than serum or plasma and rigorous validation by clinical laboratories are required. Guidelines are required to expand DBS testing and improve access to diagnostics.
About the Authors
Susan Zneimer, PhD, holds a doctorate degree in the field of genetics, is a board-certified cytogeneticist by the American Board of Medical Genetics and is a fellow of the American College of Medical Genetics. She has been a director of clinical genetic laboratories for over 20 years and is currently the co-CEO of Symbiotica and CEO of MOSYS Consulting, a company that offers clinical genetics laboratory directorship and consulting services to laboratories for clinical case review in molecular genetics and cytogenetics, laboratory expertise for quality improvement and cost reduction through Six Sigma. Zneimer has numerous publications in the field of genetics and has also published two medical reference books on genetics, Cytogenetic Abnormalities: Chromosomal, FISH and Microarray-Based Clinical Reporting (2014), and Cytogenetic Laboratory Management (2016) by Wiley Publishers.
Donna Hongo, PhD, has been in the clinical laboratory industry for more than 15 years and holds a doctorate in molecular cell biology and genetics. Hongo is a licensed clinical laboratory scientist with the state of California, and her experience includes hospital-based, emergency care facilities, reference laboratories, and molecular genetics laboratories. She is an experienced executive and has expertise in various aspects of laboratory operations and regulatory compliance. Hongo is the principal for her company Hongo Consulting, providing services and guidance to companies in the biotech and healthcare industry.
Maite Sabalza, PhD, is the scientific affairs manager at EUROIMMUN US. Her academic background is in infectious diseases and diagnostics. In her role at EUROIMMUN US, she establishes relationships with key opinion leaders and supports the team with commercial activities, including scientific collaborations, scientific marketing, and business development of diagnostics.
Ilana Heckler, PhD, is the scientific affairs associate at EUROIMMUN US. She holds a PhD in chemical biology for her studies on bacterial hemoprotein sensors of nitric oxide. As the scientific affairs associate, Heckler supports scientific collaborations and assists in the validation of diagnostic assays for autoimmune and infectious diseases
References
1. Whittaker K, Mao YQ, Lin Y, et al. Dried blood sample analysis by antibody array across the total testing process. Sci Rep. 2021;11(1):20549.
2. Blaurock G, Rische H, Rohne K. [Development of the blotting paper method in the dried blood reaction for syphilis]. Dtsch Gesundheitsw. 1950;5(15):462-464.
3.PerkinElmer, Genomics. Newborn screening. https://www.perkinelmergenomics.com/india/patients-parents/newborn-screening/#:~:text=Why%20PerkinElmer%20Genomics&text=PerkinElmer%20has%20screened%20more%20than,of%20facing%20disability%20or%20death. Accessed February on 10th, 2022.
4. Amini F, Auma E, Hsia Y, et al. Reliability of dried blood spot (DBS) cards in antibody measurement: A systematic review. PLOS ONE. 2021;16(3):e0248218.
5. McDade TW, Williams S, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007;44(4):899-925.
6. McDade TW, Burhop J, Dohnal J. High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin Chem. 2004;50(3):652-654.
7. Gupta K, Mahajan R. Applications and Diagnostic Potential of Dried Blood Spots. Int J Appl Basic Med Res. 2018;8(1):1-2.
8. Pennings JL, Siljee JE, Imholz S, et al. Comparison of different blood collection, sample matrix, and immunoassay methods in a prenatal screening setting. Dis Markers. 2014;2014:509821.
9. Buowari O. Complications of venepuncture. Advances in Bioscience and Biotechnology 2013;4.
10. Lim MD. Dried Blood Spots for Global Health Diagnostics and Surveillance: Opportunities and Challenges. Am J Trop Med Hyg. 2018;99(2):256-265.
11. Zakaria R, Allen KJ, Koplin JJ, Roche P, Greaves RF. Advantages and Challenges of Dried Blood Spot Analysis by Mass Spectrometry Across the Total Testing Process. Ejifcc. 2016;27(4):288-317.
12.PerkinElmer. Sample Collection Devices. https://www.perkinelmer.com/category/sample-collection-devices-research. Accessed February on 10th, 2022.
13. Zava TT, Zava DT. Validation of dried blood spot sample modifications to two commercially available COVID-19 IgG antibody immunoassays. Bioanalysis. 2021;13(1):13-28.
14.FDA. EMERGENCY USE AUTHORIZATION (EUA) SUMMARY COVID-19 SELFCOLLECTED ANTIBODY TEST SYSTEM (Symbiotica, Inc.) Web site. https://www.fda.gov/media/147368/download. Published 2021. Accessed February on 10th, 2022.