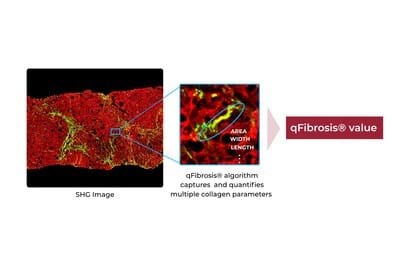
The CLIA-lab test marks HistoIndex’s expansion from research to routine diagnostics, offering AI-based digital pathology for quantitative liver fibrosis assessment in patients with MASH.
HistoIndex is returning to The Liver Meeting 2025, hosted by the American Association for the Study of Liver Diseases (AASLD), where it will highlight the recent launch of its FibroSIGHT Plus, a laboratory-developed test (LDT) for quantitative liver fibrosis assessment. The launch marks the company’s expansion from clinical trials and research into routine clinical diagnostics for metabolically dysregulated hepatosteatosis (MASH).
The test is provided from a CLIA-accredited laboratory in the US and uses AI-based digital pathology technology to deliver enhanced diagnostics for patient management in MASH therapeutics.
“We’re excited to bring the same standardization and fine-grained resolution of fibrosis measurements that enabled many therapeutic insights in MASH clinical trials, into everyday clinical care, enabling more precise liver diagnostics and better outcomes for MASH patients,” says Dr Yukti Choudhury, chief development officer at HistoIndex, in a release.
Technology Platform Expands Applications
The Singapore-based company will showcase the new test at The Liver Meeting 2025 from Nov 7-11 in Washington DC and will present one oral and 10 poster presentations, including two posters of distinction and one late-breaking poster.
“Our work at AASLD 2025 shows how our technology advances AI-based digital pathology assessments in liver disease and beyond. From detecting nuanced treatment effects in advanced MASH fibrosis and cirrhosis to continuing to expand into AATD, inflammation, vascular assessment, and MASH-HCC models, these studies highlight the wide clinical applications of our technology,” says Dr Dean Tai, chief scientific officer at HistoIndex, in a release.
Clinical Validation Study
At the conference, Dr Naim Alkhouri, chief academic officer at Summit Clinical Research and director of the Steatotic Liver Disease Program at the Clinical Research Institute of Ohio, will present new data comparing quantitative AI-based analysis to pathologists’ assessment of fibrosis.
The findings will address limitations of non-invasive tests and standard pathology in identifying target populations for MASH treatment and potential impact on patient management.
HistoIndex will also highlight its collaboration with the National Institutes of Health and Virginia Commonwealth University, where stain-free Second Harmonic Generation and AI analytics are being applied to the NASH-CRN cohort to identify fibrosis trajectories.
Photo caption: FibroSIGHT Plus
Photo credit: HistoIndex