The GeneSight psychotropic test, a pharmacogenomic test from Myriad Genetics, Salt Lake City, has been shown to help improve clinical outcomes for patients taking medications with gene-drug interactions for depressive disorders.
The observations resulted from a new analysis of the recent Genomics Used to Improve Depression Decisions (Guided) clinical trial to help improve clinical outcomes, including remission, response, and symptoms.1,2 Improvement in all three endpoints was statistically significant.
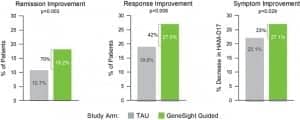
Figure 1. GeneSight helped to provide significant improvements in all patient outcomes. Graphic courtesy Myriad Genetics.
“Treatment decisions for people with major depressive disorder are challenging, particularly for patients who have already not benefitted from one or more medications,” says Michael E. Thase, MD, professor of psychiatry at the Perelman School of Medicine at the University of Pennsylvania and lead author of the study. “In some cases, nonresponse or intolerance to a standard antidepressant can result from a gene-drug interaction. When that’s the case, you want to select a different medication that doesn’t have that interaction.”
Patients enrolled in the Guided clinical trial were diagnosed with major depressive disorder and had failed at least one psychotropic medication. Patients were randomized to treatment as usual or to the GeneSight guided-care arm, in which clinicians had access to the GeneSight test report to inform their medication decisions.
The analysis evaluated 787 people from the Guided clinical trial who were taking medications with gene-drug interactions at baseline, as identified by the GeneSight test.
The results showed that people in the GeneSight arm (n = 357) achieved statistically significant improvements in all clinical outcomes compared to those in treatment as usual (n = 430) at week 8 (Figure 1).
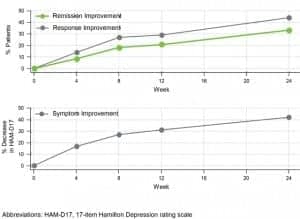
Figure 2. Durability of improvements in patient outcomes through week 24 for patients in the guided-care arm who were taking medications with predicted gene-drug interactions at baseline. GeneSight clinical benefits were durable and improved over time. Graphic courtesy Myriad Genetics.
Importantly, the improvements in all clinical outcomes were durable and continued throughout the 6-month follow-up period (Figure 2). From week 8 to week 24, remission increased 82%, response rates increased 64%, and symptom improvement increased 56%.
“Patient outcomes in the GeneSight-guided arm were superior to treatment as usual. Additionally, patients continued to get better over time and the rate of remission, which is the goal of treatment, nearly doubled from week 8 to week 24,” says Thase. “Too often patients with difficult-to-treat depression relapse, and this data shows that the patient benefits in the GeneSight-guided arm were durable.”
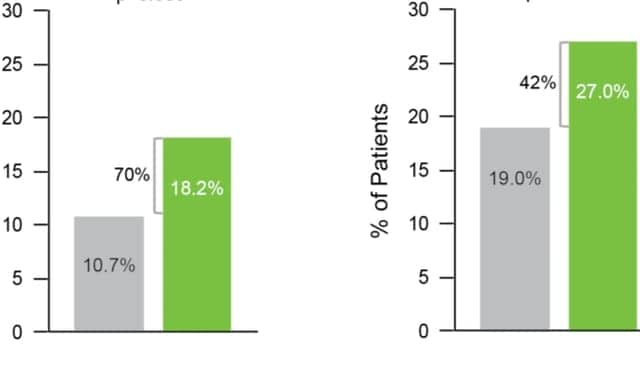
The new analysis also assessed how the GeneSight test affected outcomes for patients who were taking medications with gene-drug interactions at baseline and who switched medications, which was defined as dropping at least one medication and adding at least one different medication (Figure 3). Among people who switched, all clinical outcomes were statistically significantly better for those in the GeneSight arm (n = 235) compared to those in treatment as usual (n = 225) at week 8, underscoring the value of using combinatorial pharmacogenomic information to help inform treatment decisions.

Figure 3. GeneSight helped to improve outcomes for patients switching medications. Graphic courtesy Myriad Genetics.
“The GeneSight test identified patients taking medications with gene-drug interactions, and physicians were able to use this information to switch patients to medications with fewer gene-drug interactions and improve their outcomes,” says Michael R. Jablonski, PhD, vice president of medical affairs at Myriad Neuroscience. “This is an important first step in the journey toward making precision medicine a reality for people suffering from depression.”
For more information, visit Myriad Genetics.
References
- Thase ME, Parikh SV, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes for patients taking medications with gene-drug interactions in a randomized controlled trial. J Clin Psychiatry. 2019;80(6):19m12910; doi: 10.4088/jcp.19m12910.
- Greden JF, Parikh SV, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the Guided trial: a large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res. 2019;111:59–67; doi: 10.1016/j.jpsychires.2019.01.003.