Advanced technologies help blood banks achieve higher standards
By Gary Tufel
According to the American Red Cross, nearly 5 million people in the United States receive blood transfusions each year. The principal sources of the blood used in such procedures are blood banks—organizations that collect blood from donors and separate it into its various components, so that each component can be used most effectively to meet the needs of patients.
According to the American Society of Hematology, the first US blood blank was established in 1936. Since then, blood banking—the process of collecting, separating, and storing blood—has become a critically important function in the US healthcare system. (For more information, visit the companion article, “Stumping for a Better Match.”) Although advanced drugs can help people’s bone marrow produce new blood cells more rapidly, the body’s response time can still take weeks, so donated blood processed by blood banks remains an important and immediate life-saving resource.
Today, both private and institutional blood banks and biobanks are attracting significant amounts of investment, but they’re also facing challenges. Maria Thompson, PhD, vice president of scientific affairs at BioCision LLC, San Rafael, Calif, a supplier of tools designed to standardize blood banking and laboratory processes, says that many of the problems faced by blood banks also confront other areas of biobanking, including standardizing protocols for sample handling.
Blood banks face a three-pronged challenge, says Kevin Jones, PhD, senior director for global sales and marketing at in vitro diagnostic developer MedMira Inc, Halifax, NS, Canada. “Aside from the critical shortage of qualified personnel faced by labs in many disciplines, and the urgent need to keep the blood supply safe (also faced by labs involved in tissue banking and with other samples), there’s also a big need for blood banks to control costs,” says Jones.
To address such issues, notes Thompson, “In the past few years, there’s been a heavy increase in self-regulation on the part of biobanks, establishing and strengthening stringent guidelines in the biospecimen and blood banking sample handling environment.”
FROM HERE TO THERE
In spite of such efforts, however, many biospecimen banks are still challenged by the fact that they can’t control how samples are handled, stored, and transported to them. “Biobanks don’t always know what happens to samples from the time they’re drawn from a patient to the time they arrive at the bank,” says Thompson. “Were they shipped at room temperature or at 4°C? It matters—and it can have safety and efficacy consequences.”
A very common challenge related to the blood supply chain has to do with sample quality. ”If there is a weak link in the chain, this could result in a complete loss of sample at any given time,” says Matthew Hamilton, president of Hamilton Storage Technologies, Franklin, Mass. “Given that sample supply chains can be quite long and complex, this poses a substantial risk to any organization.”
“Shipping, handling, and storage of samples is a very big deal, and should not be overlooked,” agrees Thompson. She cites the example of a study conducted by Australia’s Mental Health Research Institute, which demonstrated the effects of poor sample handling on blood-based diagnostic markers for Alzheimer’s disease. In the study, variations in results were attributed to the fact that samples had been handled, processed, and stored at varying temperatures.
Poor storage protocols in blood and biobanks can bring about major consequences, Thompson says, particularly in the first and last miles of the collection chain—that is, how samples make it to the biobank and how they ultimately get to the researcher. “This part of the process is not as closely regulated as it should be, and errors can cause misdiagnoses and other problems,” Thompson explains. “It’s like a break in the chain.”
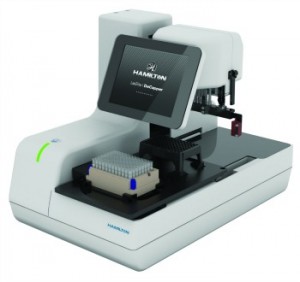
The LabElite I.D. Capper by Hamilton Storage Technologies combines decapping/capping and high-speed 2D barcode reading in a single device.
Hamilton says that his company’s LabElite line of benchtop devices offers labs an easy and efficient way to automatically process and track samples while ensuring sample integrity. “The LabElite I.D. Capper enables labs to combine decapping/capping and high-speed 2D barcode reading in a single device, without additional user interaction,” he says. “The device is specifically designed to eliminate sample contamination and assist with sample tracking during end-to-end automated liquid handling and processing. LabElite devices quickly process all common labware types, providing a flexible solution for optimizing workflows.”
COOL, STANDARDIZED
In cell therapy, blood is drawn from a patient, cells are isolated and modified, and the modified cells are then injected back into the patient. This process effectively makes the modified cells a “drug product,” and therefore subject to regulation by the FDA Center for Biologics Evaluation and Research.
“FDA wants to make sure that there are tight regulations and SOPs documenting every step in how biospecimens are handled—from the patient, to the lab, to storage, and back to the patient,” says Thompson.
The move toward greater standardization is being led mostly by commercial and clinical organizations that have had long experience in dealing with regulated processes—and with FDA. Turning the usual flow of discovery and implementation on its head, in this movement academic researchers are followers, not leaders, learning about the tools and tricks of standardization from companies that know them well. “The tools that bring standardization to the clinic will serve the same purpose in research labs,” says Thompson, “resulting in more reliable results and a faster pace of research, as more techniques become automated.”
Standardizing technologies will change the way biomedical research is done, as they move from industrial and clinic settings into research labs. In other words, cell therapy has a real chance to improve standardization and reproducibility, not just in development but also in basic research.

BioCision’s CoolCell alcohol-free cell freezing container freezes samples at 1° per minute, ensuring standardization and accuracy.
To that end, says Thompson, BioCision offers such products as CoolCell cell-freezing containers, controlled-rate cell-freezing systems that ensure standardized and reproducible cell cryopreservation. “For biobanks that acquire only a handful of samples in a week and don’t need a large and expensive electronically controlled freezer, the CoolCell freezing container is a cost- and space-saving solution, says Thompson. “And it’s portable, so it can be utilized in different areas of the lab.
“Adopting the CoolCell device in the lab is an excellent way to standardize cell freezing,” she adds. “It compares favorably with other controlled-rate freezers, which can cost around $20,000 and come with ongoing maintenance costs that soon mount up.”
SCREENING OUT RISK
Safely collecting, transporting, and storing samples are major challenges, agrees MedMira’s Jones. And the risks involved in such processes are equally real: for example, the risk of typhus that is associated with tissue collecting, and the risk of human immunodeficiency virus (HIV) when handling infected blood samples.
A great deal of current donor testing is devoted to looking for hepatitis B, hepatitis C, and HIV. “To reduce risks, it’s vital to distinguish the acceptable donors from the larger population of those who want to donate,” says Jones. “Blood banks must currently screen and monitor those who provide samples in order to assess the lifestyle, and thus the risk potential, of each donor.”
But to be practical, such high-risk testing should be simple, quick, and effective at finding donors who are risk-free, Jones says. And it should also be cost-effective.
“It comes down to managing resources,” he says.
“The cost of a normal contingent of three tissue-bank techs working for a couple days would be astronomical compared to typical blood-banking costs,” says Jones. “But the rationale is the same in both cases. Blood banks must conduct testing to ensure that the nation’s blood supply is safe. But the need for more rigorous testing coupled with a dearth of qualified people has sent blood-bank costs spiraling upward.”
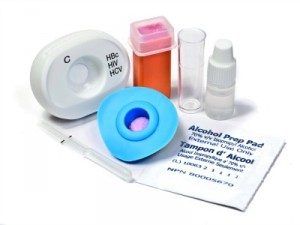
Currently undergoing clinical studies, the Multiplo HbC/HIV/HCV test by MedMira detects blood-borne viruses and provides results in about 2 minutes.
MedMira has developed a new 2-minute test—not yet on the market—which requires just one drop of blood and offers results almost instantly. During the recent annual meeting of the American Association for Clinical Chemistry, the company showcased its technology in two multiplexed versions—the Multiplo syphilis/HIV test, and the Multiplo hemoglobin C/HIV/HCV test—both of which are currently undergoing clinical studies, with an anticipated launch sometime next year.
“The test provides an instant yes or no for blood-borne viruses, and it doesn’t automatically dismiss potential donors because of their lifestyles,” says Jones. “No test is 100% accurate, but this new test significantly reduces time and cost, especially in comparison to the 24 hours typically required for blood bank testing.”
Developed on a contract with the US Department of Defense, the test is now working its way through the FDA approval process. When it becomes available, early next year, the test is expected to offer enormous benefits for blood testing in combat situations, where testing is difficult and time is especially of the essence.
PERSONALIZED THAWING
Advances in genomics have given rise to such new disciplines as pharmacogenomics and metabolomics, where researchers are testing huge numbers of biological specimens in order to classify subpopulations with specific genomic predispositions.
“The goal is to personalize medicine by better matching drugs and dosage to individual patient profiles,” says Hamilton. “By doing this, manufacturers might be able to keep on the market a blockbuster drug that would otherwise have been withdrawn because of adverse effects on just a few individuals.”
In the course of conducting research on such large groups of specimens, investigators will inevitably need to process frozen samples. Having in place a standard procedure for thawing specimens will be critical to protecting the integrity of “omics” studies.
Traditional methods of specimen thawing, such as swirling samples in water baths or warming samples between hands, are unsuited for processes that require consistency. Equally important, they lead to subjective methods of gauging thawing, such as visually examining samples for the last traces of ice crystal.
According to Thompson, BioCision’s support of industry standardization includes a controlled thawing solution to eliminate “user technique” and subjectivity from this critical part of the workflow, while also ensuring that cell viability remains high and sample integrity is intact. The company’s ThawStar device is an automated platform for thawing cryogenic vials in a standardized manner, addressing the final stage in the cryopreservation workflow.
“Someday we might not have to freeze samples,” says Thompson. “But so long as we do, we need to improve consistency and reduce sample variability.”
Beyond research applications, timely processing of frozen cells can also be an important element of patient care. In 2007, procedural protocols were overhauled to require that peripheral blood mononucleated cells—cryopreserved cells derived from blood samples and used to treat cancer, HIV, and other dangerous illnesses—be used within 8 hours of being drawn from a patient’s vein.
SETTING BEST PRACTICES
“As protocols and methods change, education is the key to keeping pace,” says Thompson. “BioCision keeps in touch with clients to update them, handhold, and troubleshoot when necessary. But some labs and lab personnel are still using legacy protocols that are more than 20 years old. Industry needs to keep current and not lose sight of the patient as the ultimate end user.”
MedMira’s Jones agrees that the blood supply issue remains challenging. “The main concerns are avoiding infection in the blood supply, reducing the number of samples that need to be disposed of, speeding the testing of samples, making the process more efficient, and reducing costs,” he says.
But there is also a need for greater sharing of information, says Jones. And in the case of collections performed by private companies, there is a need to figure out how to set best practices.
Follow-ups and careful monitoring of infection in transplants and other areas are a function of self-regulation and strict control. But for the purposes of safeguarding the blood supply, says Jones, regulation is not the enemy.
“It’s just sensible to keep the blood supply safe,” he says. “Industry does not want to risk in any way having a contaminated blood supply getting past safeguards, and the best way to guard against that is to cooperate and share knowledge.”
For more information, visit the companion article, “Stumping for a Better Match.”
Gary Tufel is a contributing writer for CLP. For further information, contact CLP chief editor Steve Halasey via [email protected].