New clearances expand MALDI-TOF platform capabilities with improved software, faster sample prep, and broader species coverage.
The US Food and Drug Administration (FDA) has cleared two additional claims for Bruker’s MALDI Biotyper CA System, expanding the mass spectrometry platform’s diagnostic capabilities for clinical microbial identification.
The clearances include enhanced software and hardware components (Claim 7) and an expanded reference library (Claim 8) that increases validated species coverage to 549 microbial species across gram-positive and gram-negative bacteria, anaerobes, and yeasts.
Bruker announced the FDA clearances at the annual IDWeek meeting in Atlanta.
Software and Hardware Enhancements
Claim 7 covers the MBT Compass HT CA software and MBT FAST Shuttle US IVD components. The updated software provides parallel data processing, improved user management, and support for 21 CFR Part 11 compliance. It includes IDealTune, an automated tuning function that maintains system performance by continuously monitoring US IVD Bacterial Test Standard quality control results, reducing manual tune-up requirements.
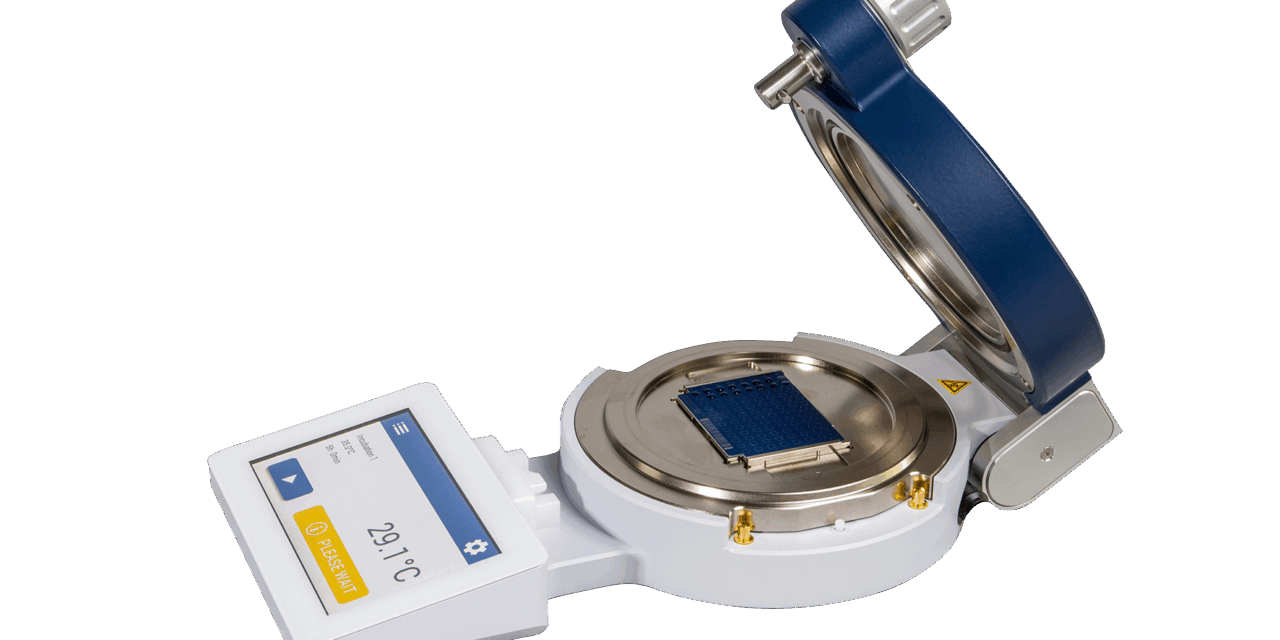
The MBT FAST Shuttle US IVD accelerates sample preparation by enabling faster drying of droplets, including MALDI matrix, compared to room temperature drying. The component also standardizes sample preparation to produce more consistent matrix crystallization, which can improve identification accuracy.
The MALDI Biotyper CA System uses MALDI-TOF technology for rapid microorganism identification from isolated colonies or positively flagged blood cultures following culture from human specimens.
Expanded Species Library
Claim 8 encompasses the expanded FDA-cleared reference library, which now contains reference spectra for 549 clinically validated microbial species organized into 437 groups. The system also includes more than 3,400 non-clinically validated species, which are clearly marked in reports and not transmitted to laboratory information systems.
Implementation Timeline
Bruker is finalizing internal processes to prepare for shipment of the updated system in the US and Puerto Rico. Current MALDI Biotyper CA System users can upgrade their existing systems with the newly cleared features, including the updated reference library, MBT FAST Shuttle US IVD, and MBT Compass HT CA software.
“This FDA clearance represents a major step forward in our mission to support clinical laboratories with faster and more reliable microbial identification,” says Carla Schneider, director of commercial operations for the Americas at Bruker Microbiology & Infection Diagnostics, in a release. “The MBT Compass HT CA software and MBT FAST Shuttle US IVD not only streamline workflows and improve operational efficiency, but also open the door for future claims and innovations.”
Photo caption: MBT FAST Shuttle US IVD
Photo credit: Bruker