Modifications to an NGS pipeline can improve the utility of archived specimens
By Brian Walker, PhD, and Elisa Izquierdo Delgado
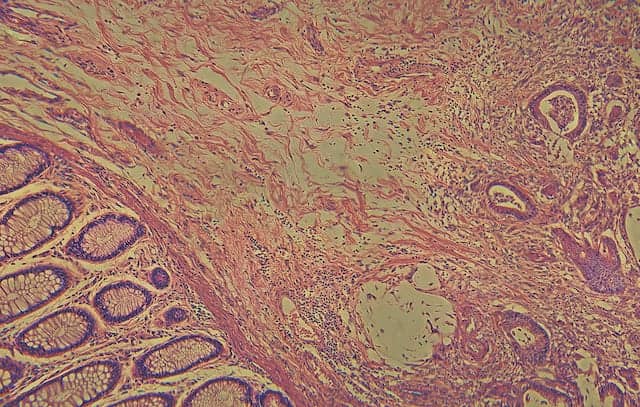
One of the most widely used ways to preserve and archive clinical specimens is the preparation of formalin-fixed, paraffin-embedded (FFPE) samples. Such archived specimens represent a valuable source of tissue for molecular studies. However, ensuring good-quality genetic sequencing data from DNA that has been extracted from FFPE specimens can be challenging due to both quality and quantity issues (see Figure 1).
With as many as 1 billion FFPE samples in storage around the world, it is essential to guarantee a robust and reproducible protocol for delivering sequencing results.1 To accomplish this goal, the Center for Molecular Pathology at the Royal Marsden Hospital, London, recently optimized a targeted next-generation sequencing (NGS) method using clinical FFPE samples.
The Royal Marsden Hospital and its associated Institute of Cancer Research together represent Europe’s largest comprehensive cancer center, serving more than 50,000 patients annually. The Center for Molecular Pathology, a joint initiative between these institutions, opened in 2012 with the mission of improving personalized treatment for cancer patients.2 As part of that work, a team of researchers developed a number of targeted NGS panels for specific types of cancer, making it possible to provide clinicians with an in-depth view of the genetic variations in a tumor.
When these efforts began, however, the turnaround time for reporting NGS panel results was too long to have an effect on treatment selection. Also, many patients seen by the hospital’s medical staff had biopsied tissues stored in FFPE format, making the specimens unsuitable for sequencing with the center’s traditional NGS protocol.
Through an intensive optimization process, the center’s scientific team was able to improve the library construction method for FFPE samples, and consistently deliver reliable sequencing data from such samples in a clinically relevant time frame. The pipeline established at the Center for Molecular Pathology can be used in any clinical laboratory dealing with similar challenges in suboptimal sample quality.
In this article, we report on the modifications introduced to create the center’s method, and how they have enabled routine NGS analysis of degraded FFPE samples for clinical research.
CANCER PANELS
With next-generation sequencing, scientists can test many genes more rapidly and cost-effectively than was possible with methods based on polymerase chain reaction (PCR), Sanger sequencing, or other types of assays commonly used in clinical laboratories.3 The first NGS panels used in the Royal Marsden center were designed for pediatric cancers and cancers of the blood, breast, gastrointestinal tract, and lung. Each panel targets several genes known to be altered in that type of cancer, and provides for the detection of copy number variations, insertion or deletion mutations (indels), single-nucleotide polymorphisms (SNPs), and structural rearrangements, as well as wild-type status.

Figure 1. A series of FFPE samples showing varying levels of quality. FFPE samples are often highly degraded due to the fixation process, resulting in low-quality material that is not ideal for many scientific workflows. Click to expand.
It’s important to deliver a comprehensive report with high confidence for each of the genes analyzed in the panels. Many studies have demonstrated that genetic variations—whether a single base change, a large structural variant, or copies of a whole gene—have implications for tumor behavior and response to treatment. Wild-type status can also be useful information: in some gastrointestinal cancers the status of KRAS or NRAS is crucial for predicting a patient’s response to epidermal growth factor receptor (EGFR) inhibitors.4
At the Center for Molecular Pathology, the NGS protocol was designed for the MiSeq NGS platform by Illumina, San Diego, which offers an affordable option for sequencing, even at the low throughput characteristic of most clinical lab pipelines. Sequencing data from the MiSeq platform enables scientists to analyze and generate a report using a desktop computer, with little need for a complex bioinformatics infrastructure.
FFPE SAMPLE PROCESSING
A key goal among oncologists is the application of NGS technology to clinical samples in order to provide genetic analysis as a guide for personalized medicine. Unfortunately, samples available for use in clinical diagnostics are often in the form of archival FFPE tissue—a format that is well suited for preserving tissue morphology, for pathology diagnosis, and for long-term storage—but a format that also raises other challenges. For instance, it is well known that tissue fixation using formalin affects nucleic acid quality, leading to DNA deamination and fragmentation.5 In addition, samples are often available only as small-core needle biopsies, providing low concentrations of DNA.

Figure 2. Based on the same amount of sequencing, Kapa HyperPlus kits provided superior coverage depth compared to a standard protocol for Covaris shearing. This graphic shows coverage at 250x. For the three FFPE samples, HyperPlus coverage at 250x was better than 80%, while Covaris coverage averaged 73.7%. For the control blood samples, average HyperPlus coverage was 86.2%, and Covaris was 65.7%. Click to expand.
Such challenges were not of much concern until the era of molecular genetics. For decades, the vast majority of what was known about cancer was based on cell morphology. Only in recent years has our understanding of the genetic basis of cancer become reliable enough to make DNA analysis of tumors a standard requirement along with histopathology.
The biomedical community will not be leaving FFPE processing behind anytime soon. For now, it falls to the researchers conducting DNA analysis to find more robust methods for getting high-quality results from this sample type. Challenges include accurately assessing the quality and quantity of DNA input prior to library construction.
The Center for Molecular Pathology team evaluated a number of options to improve the FFPE sample processing workflow. Since there was no way to improve the quality of the DNA coming in, solutions to the problem would need to work well on the archival and degraded DNA typically seen in clinical laboratories.
A critical step was the adoption of strict quality control measures to eliminate the processing of samples that did not meet minimum requirements for DNA quantity. Attempting to prepare and sequence such samples would be wasteful, as they would not lead to reliable data. Both TapeStation from Agilent Technologies, Santa Clara, Calif, and Qubit from Thermo Fisher, Waltham, Mass, provide information about DNA fragmentation and quantity that make it possible to determine at the start whether a sample will yield enough material for successful sequencing.6,7
Another useful approach involved switching fragmentation and library construction to a single-tube system from Kapa Biosystems, Wilmington, Mass. The Kapa HyperPlus kit employs a novel cocktail for enzymatic fragmentation, which is gentler on the sample, takes less time than mechanical shearing, and introduces minimal fragmentation bias (see Figure 2).8 The kit’s chemistry allows for the conversion of more input DNA to an adapter-ligated library, which is critical for achieving high library complexity from challenging FFPE samples prior to target enrichment.
Ultimately, the HyperPlus kit delivered more unique molecules and a higher yield of DNA leading into the PCR step. Libraries prepared this way were more diverse and required fewer PCR cycles, reducing the number of duplicates, achieving a higher yield, and providing more uniform sequencing coverage compared to libraries processed with shearing DNA protocols (see Figure 3).

Figure 3. In a comparison of Kapa HyperPlus kit performance to standard Covaris shearing, duplication rates found after sequencing were lower for most libraries prepared with HyperPlus. The average duplication rate for FFPE libraries with HyperPlus was 60%, a 26.3% decrease compared to the average rate for the Covaris method. Click to expand.
A final step that was critical for generating high-quality libraries from low-input samples was the use of capture reagents from NimbleGen Roche, Madison, Wisc, which allowed the pooling of samples prior to hybridization.9 This step reduced the number of baits needed, keeping costs down. Because this step meant attaching adapters earlier in the process, it also helped to reduce the chances of cross-contamination between samples.
Together, these adjustments to the NGS pipeline made it possible to prepare and sequence FFPE samples as robustly as any other type of DNA sample, routinely generating results of the quality needed for clinical reporting. The modifications improved the sensitivity and specificity of testing, as well as the reproducibility of results. Indeed, analyses comparing fresh frozen samples against FFPE samples have shown that despite the wide range of FFPE sample quality, single-nucleotide variants and copy number changes can be accurately detected in FFPE as well as in fresh frozen samples.
This method substantially expands the pool of samples that can be processed in the clinical lab. The modified pipeline also significantly reduces the amount of time needed to sequence and analyze FFPE samples, which was essential for reporting results in a clinically relevant time frame.
CLINICAL RESULTS
Two of the NGS panels developed at the Center for Molecular Pathology have gone into clinical trials for breast and gastrointestinal cancers, and a pediatric panel is now routinely testing patients with solid tumors in a feasibility study for the incorporation of a future multiarm trial. The gastrointestinal study is expected to recruit about 250 patients whose tumors and matched normal samples are being evaluated with a 46-gene panel. Results from that analysis are being incorporated into the healthcare process, using genetic results to recommend more personalized treatment courses for each patient.

Figure 4. A step-by-step illustration of the workflow using Kapa HyperPlus kits with estimates of time involved, ending with paired-end sequencing. The process was sufficiently shortened that results could be generated within a clinically actionable timeframe. Click to expand.
The center’s original plans for clinical genomics did not include collection of matched normal samples from patients. However, comparing genomic variants found in tumor DNA to a peripheral blood sample is one of the fastest and most cost-effective ways to determine whether the variants are somatic or germline.10 Without a sophisticated bioinformatics pipeline, this process can provide information that is pivotal to downstream interpretation of variants, such as filtering out otherwise suspicious-looking variants that might simply be private SNPs for any given patient.
When the gastrointestinal clinical study began, turnaround time for sequencing results was more than 5 months—much longer than doctors could wait to make treatment decisions. The FFPE pipeline optimization steps, as well as stronger organization for acquiring samples from other hospitals, has led to a significant reduction in that turnaround time. Later in the trial, results were delivered within 9 or 10 weeks, a short enough time to make a difference in the selection of treatment course (see Figure 4).
As part of the process of incorporating tumor DNA results into patient care, the molecular diagnostic lab staff and hospital clinicians began attending regular molecular tumor board meetings. These events allow everyone to discuss results together, brainstorming ideas for clinical trials or therapeutics that might be relevant based on a patient’s tumor profile. Such meetings have proven to be beneficial not only for patients, but also for the ongoing genetic education of the hospital team.
CONCLUSION
Specific modifications to the traditional NGS pipeline make it possible to prepare and process FFPE samples as robustly and efficiently as higher-quality DNA samples. Overall, this approach—in addition to carefully designed gene panel tests, shortened turnaround times, and excellent interaction between lab scientists and clinicians—will ultimately bring the benefits of DNA sequencing and personalized treatment decisions to patients fighting many different types of cancer.
Brian Walker, PhD, is director of research and professor of medicine in the myeloma institute at the University of Arkansas for Medical Sciences; Elisa Izquierdo Delgado is a doctoral candidate at the Royal Marsden Hospital and the Institute of Cancer Research, London. For further information contact CLP chief editor Steve Halasey via [email protected].
REFERENCES
- Blow N. Tissue preparation: tissue issues. Nature. 2007;448:959–962; doi: 10.1038/448959a.
- Center for Molecular Pathology [homepage online]. London: Royal Marsden NHS Foundation Trust and Institute of Cancer Research. Available at: www.royalmarsden.org/centre-molecular-pathology. Accessed October 24, 2016.
- Strom SP. Current practices and guidelines for clinical next-generation sequencing oncology testing. Cancer Biol Med. 2016;13(1):3–11; doi: 10.28092/j.issn.2095-3941.2016.0004.
- Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359(17):1757–1765; doi: 10.1056/nejmoa0804385.
- Tokuda Y, Nakamura T, Satonaka K, et al. Fundamental study on the mechanism of DNA degradation in tissues fixed in formaldehyde. J Clin Pathol. 1990; 43(9):748–751. Available at: www.ncbi.nlm.nih.gov/pmc/articles/PMC502754. Accessed: October 24, 2016.
- TapeStation system [homepage online]. Santa Clara, Calif: Agilent Technologies, 2016. Available at: www.genomics.agilent.com/en/tapestation-system/4200-tapestation-instrument/?cid=ag-pt-181&tabid=prod2420037. Accessed October 24, 2016.
- Qubit fluorometric quantitation [homepage online]. Waltham, Mass: Thermo Fisher Scientific, 2016. Available at: www.thermofisher.com/us/en/home/industrial/spectroscopy-elemental-isotope-analysis/molecular-spectroscopy/fluorometers/qubit.html. Accessed: October 24, 2016.
- Kapa HyperPlus kits [homepage online]. Wilmington, Mass: Kapa Biosystems, 2016. Available at: www.kapabiosystems.com/product-applications/products/next-generation-sequencing-2/dna-library-preparation/kapa-hyperplus-kits. Accessed: October 24, 2016.
- NimbleGen SeqCap target enrichment [homepage online]. Madison, Wisc: Roche NimbleGen, 2015. Available at: sequencing.roche.com/products/nimblegen-seqcap-target-enrichment.html. Accessed: October 24, 2016.
- Jones S, Anagnostou V, Lytle K, et al. Personalized genomic analyses for cancer mutation discovery and interpretation. Sci Transl Med. 2015;7(283):283ra53; doi: 10.1126/scitranslmed.aaa7161.