Seegene Inc of Seoul, Republic of Korea, has received 510(k) clearance for its TOCE-based assay for herpes simplex virus (HSV) types 1 and 2, the company’s first product to clear FDA. Seegene plans to pursue future submissions of its multiplex real-time PCR reagents.
“This is an important milestone in support of our planned entry into the United States, the largest molecular diagnostic market in the world,” says Jong-Yoon Chun, PhD, founder, CTO, and CEO of Seegene. “After more than 10 years of extensive efforts to continually develop and commercialize novel technologies to surpass the capabilities of real-time PCR, we successfully invented MuDT technology, an ultimate multiplex real-time PCR technology that marks a new era in the molecular diagnostics industry. Through FDA approval, Seegene now intends to actively enhance its leadership in the multiplex real-time PCR diagnostic testing market.”
According to a press release, during 2015 Seegene intends to establish a new US subsidiary as part of a broader plan to increase its presence in the global molecular diagnostics market. The company also recently signed an agreement with Beckman Coulter to manufacture reagents for Beckman’s Veris molecular diagnostics system.
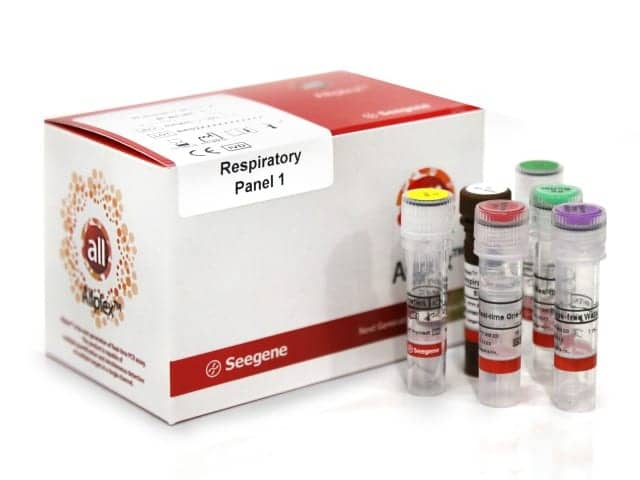
Seegene plans to continue developing its Allplex family of infectious disease panel tests for the US market. The assays are based on MuDT technology, which enables simultaneous detection and quantification of multiple targets in a single fluorescence channel, without melting curve analysis. The company’s CE-marked Allplex respiratory panel 1 simultaneously identifies and quantifies seven targets, including influenza virus A and B, RSV type A and B, and influenza A virus subtypes H1, H3, and H1pdm09. According to Seegene, the panels are the first and only high-multiplexed respiratory panels based on a real-time PCR platform.
For more information, visit Seegene.