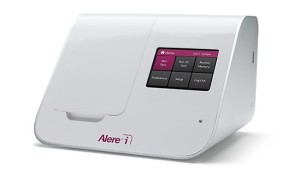
Delivers results in 8 minutes or less
The Alere i Strep A test from Alere, Waltham, Mass, detects group A Streptococcus bacteria from throat swab specimens in 8 minutes or less. Operating on the company’s Alere i platform, the isothermal nucleic acid technology amplifies at a constant temperature. Because thermal cycling and sample purification steps are not required, the test produces fast results. A multicenter study demonstrated that the test’s sensitivity is 95.9% while its specificity is 94.6% The kit features a compact footprint, intuitive touchscreen, visual guides, and unidirectional connectivity to the user’s network. The company’s platform can also run a CLIA-waived Alere i influenza A and B test, which detects and differentiates influenza A and B virus in less than 15 minutes.
Alere
(781) 647-3900; www.alere.com
Real-Time PCR Platform

Automating the entire testing process, from sample prep to amplification to real-time detection, the Cobas Liat system from Roche Diagnostics USA, Indianapolis, includes a small benchtop analyzer, an extensive array of instrument controls, and assay tubes for specific targets. The Cobas Liat analyzer compresses the assay tube to release reagents selectively from tube segments. Samples can be transferred from one segment to another, while reaction conditions can be controlled at different temperatures. As a secure and fully closed system, the system reduces the risk of cross-contamination and preserves sample integrity. Definitive, objective results are produced in 20 minutes or less. Featuring touchscreen-guided operation with minimal hands-on time, the platform can be used for the company’s Streptococcus group A and influenza A and B nucleic acid tests.
Roche Diagnostics USA
(800) 428-5076; usdiagnostics.roche.com
Melanoma Test

Myriad myPath melanoma from Myriad Genetics, Salt Lake City, is a clinically validated gene expression test designed to differentiate malignant melanoma from benign nevi across all major melanoma subtypes. The assay, which detects 23 unique biomarkers, has demonstrated high sensitivity and specificity in multiple independent cohorts. In a verification study using 464 patient samples and a separate independent validation study with 437 patient samples, the test demonstrated diagnostic accuracy greater than 90%. Acceptable samples are formalin-fixed, paraffin-embedded tissue from blocks or slides of melanocytic lesions. The assay is adjunctive and should be used in conjunction with clinical data and a pathologic work-up.
Myriad Genetics
(801) 584-3600; www.myriadpro.com/melanoma
Random Access Analyzer

GenMark Diagnostics Inc, Carlsbad, Calif, enables clinical laboratories to process multiple test types simultaneously with its eSensor XT-8 molecular diagnostics system. The instrument features a touchscreen user interface, customizable reports, and an expanding menu of multiplex in vitro diagnostic tests that can all be addressed on the same platform. The company’s respiratory virus panel requires less than 60 minutes of hands-on preparation time, including DNA extraction, and detects 14 respiratory virus types and subtypes after just over 3 hours of walk-away time. The company’s cystic fibrosis genotyping panel detects 23 mutations for which testing is recommended by the American Congress of Obstetricians and Gynecologists and the American College of Medical Genetics and Genomics. The cystic fibrosis panel requires 40 minutes of hands-on preparation time, and produces test results in approximately 3 hours. In clinical trials, the panel had 100% accuracy and reproducibility, and a 97.3% first-pass call rate.
GenMark Diagnostics
(800) 373-6767; genmarkdx.com
Postnatal Blood Test
Assists in diagnostic evaluation of intellectual disability
Affymetrix
(888) 362-2447; www.affymetrix.com
Tissue Preparation System

Using magnetic particle-based isolation and proprietary iron oxide bead technology, the tissue preparation solution from Siemens Healthcare Diagnostics Inc, Tarrytown, NY, incorporates both a tissue preparation system and Versant tissue preparation reagents. The system provides a fully automated method of isolating high-quality nucleic acids from formalin-fixed, paraffin-embedded and fresh-frozen tissues. Processing up to 48 samples in 4 hours, the system eliminates the use of organic solvents and centrifugation steps associated with deparaffinization. DNA and RNA can be extracted together using a single process with one set of reagents. Users have multiple options for protocols: total nucleic acid for DNA applications, pure RNA for RNA applications, or a combined analysis of DNA and RNA from one sample. Labs can benefit from a uniform lysis step of 1 hour for all samples, thereby optimizing reproducibility.
Siemens Healthcare Diagnostics
(800) 242-3233; www.healthcare.siemens.com
Oncology Hotspot Control
Serving as a control for assays involving more than 500 hotspot mutations, the AcroMetrix oncology hotspot from Thermo Fisher Scientific, Waltham, Mass, can be used across laboratories with different next-generation sequencing platforms, assays, and bioinformatics pipelines. Containing both synthetic and genomic DNA, the control tests precision and detects analytical deviations that may emerge from variations among reagents and instruments. It encompasses cancer-associated mutations across 53 genes, including BRAF, EGFR, ERBB2, KRAS, and TP53. Users can select from 3 complex mutations, 18 insertions, 29 deletions, and 500 single nucleotide variants. The control has a validated shelf life of 12 months at –20°C and is stable over five freeze-thaw events.
Thermo Fisher Scientific
(800) 678-5599; www.thermofisher.com
Herpes Simplex Virus Assay
Amplifies target regions within HSV 1 and 2 genomes
Qiagen
(800) 426-8157; www.qiagen.com
MicroRNA Gene Expression Classifier
Uses the expression levels of 10 miRNAs

Interpace Diagnostics
(844) 405-9655; interpacediagnostics.com
Multiplex PCR System

The FilmArray system from BioFire Diagnostics, Salt Lake City, is a multiplex polymerase chain reaction system that integrates sample preparation, amplification, detection, and analysis. Testing up to 175 samples per day, the system offers single database management of 8 instruments, bringing about high throughput with random access. Users can choose from respiratory, blood culture identification, and gastrointestinal panels, which all together test for more than 100 pathogens. The panels require 2 minutes of hands-on time, with a turnaround time of approximately 1 hour. No precise measuring or pipetting is required. The compact system also features laboratory information system connectivity.
BioFire Diagnostics
(800) 735-6544; www.biofiredx.com
Enteric Pathogens Test
2-hour run time

Nanosphere
(888) 837-4436; www.nanosphere.us
Sex-Specific Blood Test
For the evaluation of obstructive coronary artery disease

CardioDx
(866) 941-4996; www.cardiodx.com
Real-Time Strep Assay
Moderate complexity test requires 50 ?L of sample

Focus Diagnostics
(800) 838-4548; www.focusdx.com
EMT Enrichment Kit
Targets cells with epithelial to mesenchymal properties
Fluxion Biosciences
(866) 266-8380; fluxionbio.com
Saliva Purifying Collection Device
Collects two 0.5 mL saliva samples

Oasis Diagnostics
(360) 546-1563; 4saliva.com




