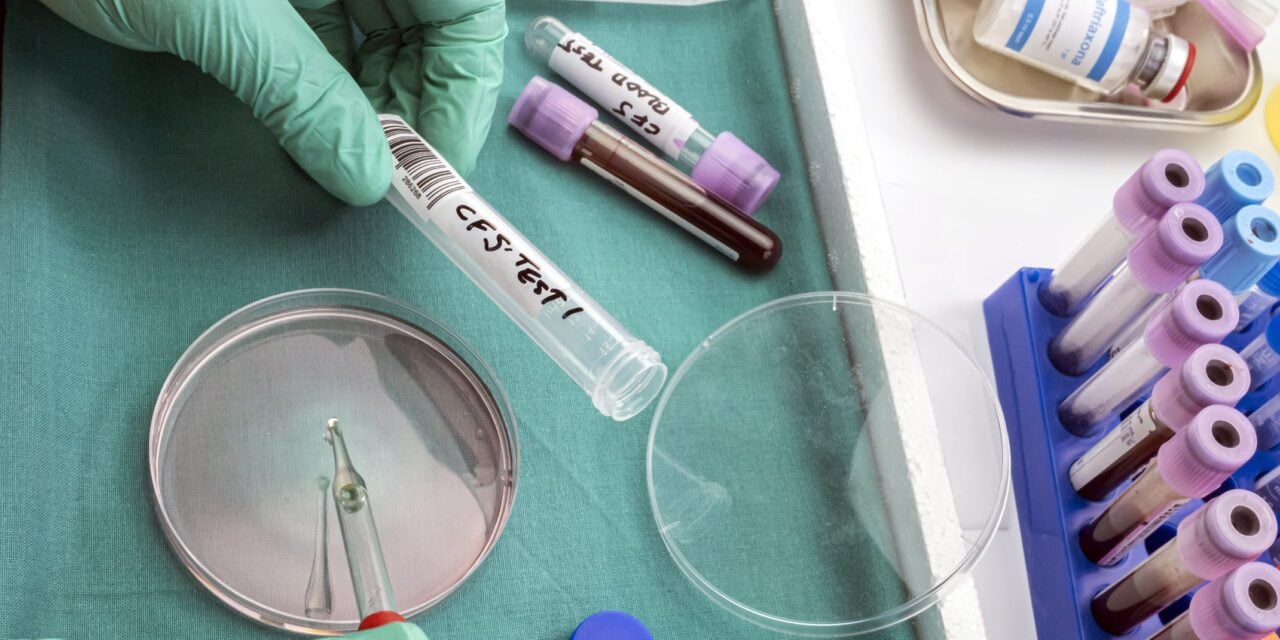
Houston-based facility meets federal regulatory requirements for cerebrospinal fluid testing to detect central nervous system metastases.
CNSide Diagnostics has received Clinical Laboratory Improvement Amendments (CLIA) accreditation from the Centers for Medicare and Medicaid Services for its clinical laboratory in Houston, Texas, clearing a key regulatory hurdle for the broad US market release of its cerebrospinal fluid assay platform.
The accreditation confirms the laboratory meets federal standards under the CLIA for testing human specimens, including requirements for proficiency testing, personnel qualifications, and quality control.
CNSide Diagnostics, a wholly owned subsidiary of Plus Therapeutics Inc, develops laboratory-developed tests designed to identify tumor cells that have metastasized to the central nervous system in patients with carcinomas and melanomas.
“This is a key milestone on our trajectory to bring the CNSide cerebrospinal fluid assay platform to the broadest possible set of patients with or at risk for CNS cancers and simultaneously underscores our commitment to the highest quality standards,” says Russ Bradley, president and general manager of CNSide Diagnostics, in a release.
The CNSide CSF Assay Platform enables quantitative analysis and molecular characterization of tumor cells and circulating tumor DNA in cerebrospinal fluid to inform management of patients with leptomeningeal metastases, according to the company.
The CLIA certification represents the latest step in the company’s US market access and launch strategy. The accreditation is necessary to achieve additional regulatory and commercial milestones for the diagnostic platform.
Plus Therapeutics, headquartered in Houston, is a clinical-stage pharmaceutical company developing targeted radiotherapeutics for central nervous system cancers. The company combines image-guided local beta radiation and targeted drug delivery approaches in its pipeline of product candidates for leptomeningeal metastases and recurrent glioblastoma.
The company has established a supply chain through strategic partnerships to enable development, manufacturing, and potential future commercialization of its products.
ID 198504490 © Felipe Caparros Cruz | Dreamstime.com