Cancer detection specialist Elypta, Stockholm, Sweden, has enrolled the first patient in the company’s multicenter Aurorax-087A study (AUR87A) involving the company’s liquid biopsy platform. The AUR87A trial will involve 16 hospitals across Europe and the United States, making it the largest-ever prospective multicenter study of a diagnostic for the detection of recurrence after surgery in clear cell renal cell carcinoma (ccRCC), the most common form of kidney cancer.
Globally, more than 322,000 people are diagnosed with ccRCC each year. Of the 70% to 80% who undergo curative surgery, 20% suffer a recurrence within 5 years, with about half within the first 2 years. Current follow-up guidelines recommend surveillance of patients using imaging at regular intervals after surgery. However, about 30% of recurrences are detected between planned visits, with patients being symptomatic and frequently having more advanced disease at recurrence detection.
Elypta’s liquid biopsy is expected to detect tumor progression more rapidly than imaging. Such earlier detection offers the possibility of saving or prolonging the lives of those suffering a recurrence.
The AUR87A trial will prospectively enroll up to 280 nonmetastatic ccRCC patients curatively treated with surgery. Blood and urine samples collected prior to and at every 3 months after surgery will be analyzed to assess glycosaminoglycan (GAG) values. The GAG scores will be compared to regular imaging assessments taken alongside blood and urine sampling as part of the patient’s follow-up schedule. The AUR87A trial aims to demonstrate that a postsurgery increase in a patient’s GAG score can detect recurrence at an earlier or equal time-point compared to imaging.
“Currently, there are no clinically applied blood or urine-based markers for kidney cancer surveillance, and there is a real need for more precise and frequent follow-up methods after curative intent surgery,” says urologist Saeed Dabestani, MD, PhD, chief principal investigator for the AUR87A study. “AUR87A is the first-ever prospective multicenter diagnostic test study (level of evidence 1B) to evaluate such a method for postoperative surveillance of patients with ccRCC.”
Elypta’s liquid biopsy platform quantifies blood- and urine-based metabolic GAG markers and then uses Cloud-based software to compute a cancer-specific score. The platform has the potential to be used in the diagnosis or monitoring of a range of cancers, of which ccRCC is the first type to be evaluated.
The company’s study is being cofunded by the European Union’s Horizon 2020 research and innovation program under grant agreement no. 849251.
For more information, visit Elypta.
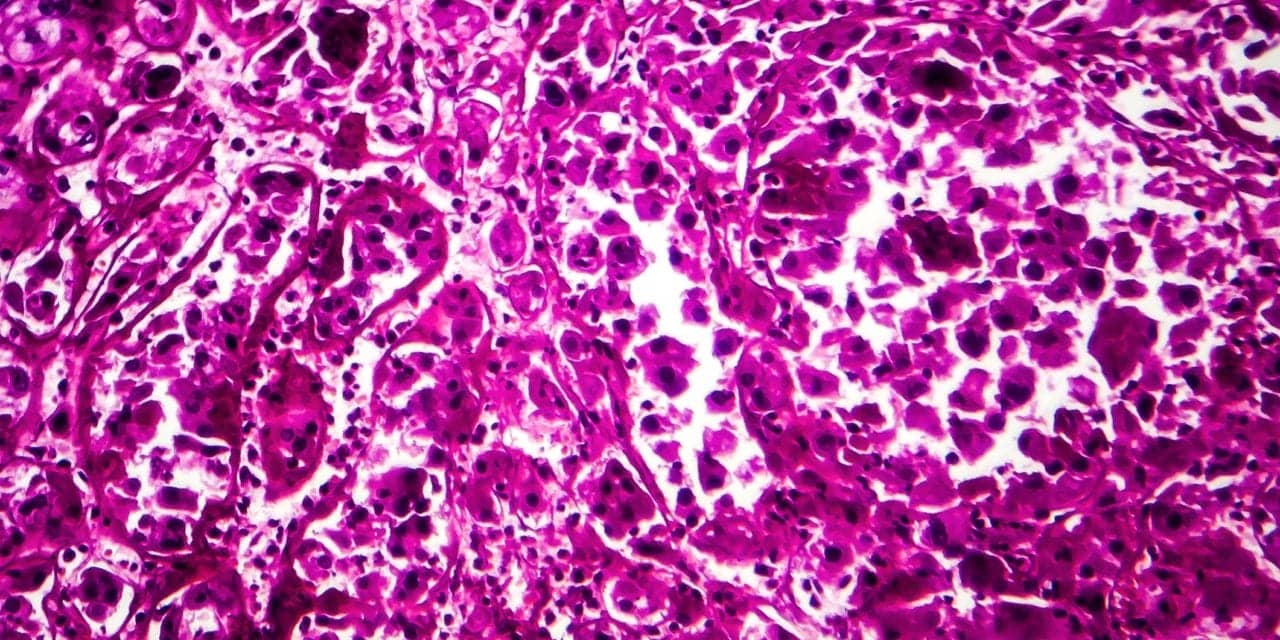
Featured image: Light micrograph of renal cancer cells. Image © Katerynakon, courtesy Dreamstime (ID 163938180).