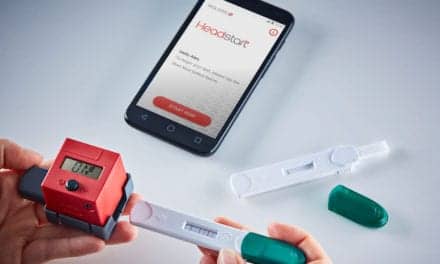
Summary: Intelligent Bio Solutions completed biocompatibility testing for its fingerprint drug screening system, advancing toward 510(k) regulatory clearance.
Takeaways:
- Successful Biocompatibility Testing: The system passed rigorous ISO 10993 standards, confirming the safety of materials used in its drug screening cartridge, confirmatory cartridge, and DSR-Plus reader for medical use.
- FDA 510(k) Pathway: This milestone is a crucial step in INBS’s plan to obtain FDA 510(k) regulatory clearance, with the 510(k) package submission expected in the fourth quarter of 2024.
- Innovative Non-Invasive Technology: The Intelligent Fingerprinting Drug Screening System represents cutting-edge, non-invasive drug testing technology, aimed at entering the U.S. market by 2025.
Intelligent Bio Solutions, a medical technology company delivering intelligent, rapid, non-invasive testing solutions, announced the successful completion of biocompatibility testing of its Intelligent Fingerprinting Drug Screening System, a pivotal phase in the clinical study plan required for FDA 510(k) regulatory clearance.
The biocompatibility study, conducted in accordance with ISO 10993 standards, involved a thorough evaluation of the system’s materials that come into contact with human skin. With the successful completion of the biocompatibility study, INBS has confirmed that all materials used in its system, including the Drug Screening Cartridge, Confirmatory Cartridge and the DSR-Plus Reader, are safe for use in medical devices.
“This milestone is an important element of our FDA 510(k) pathway for regulatory clearance, and we are proud to announce its completion,” says Harry Simeonidis, president and CEO at INBS. “Our development team continues to execute on our plan to meet FDA requirements for our Drug Screening System. We are now poised to move forward with the next stages of our clinical study plan and move closer to bringing our innovative technology to the market.”
Drug Screening Through Fingerprints
The Intelligent Fingerprinting Drug Screening System, which includes a drug screening cartridge, a DSR-Plus reader, and a collection kit for laboratory analysis, is at the forefront of non-invasive drug testing technology. The company expects to submit the 510(k) package in the fourth calendar quarter of 2024, moving closer to its goal of bringing this innovative technology to the U.S. market in 2025.
“The planned biocompatibility testing protocol included cytotoxicity, sensitization and irritation tests conducted on drug screening system materials and was presented to the FDA in November last year,” says Rafael da Luz, vice president of regulatory affairs for INBS. “Earlier this year, the FDA agreed that the proposed testing approach would be adequate for a future premarket 510(k) submission.”
Photo: Intelligent Bio Solutions