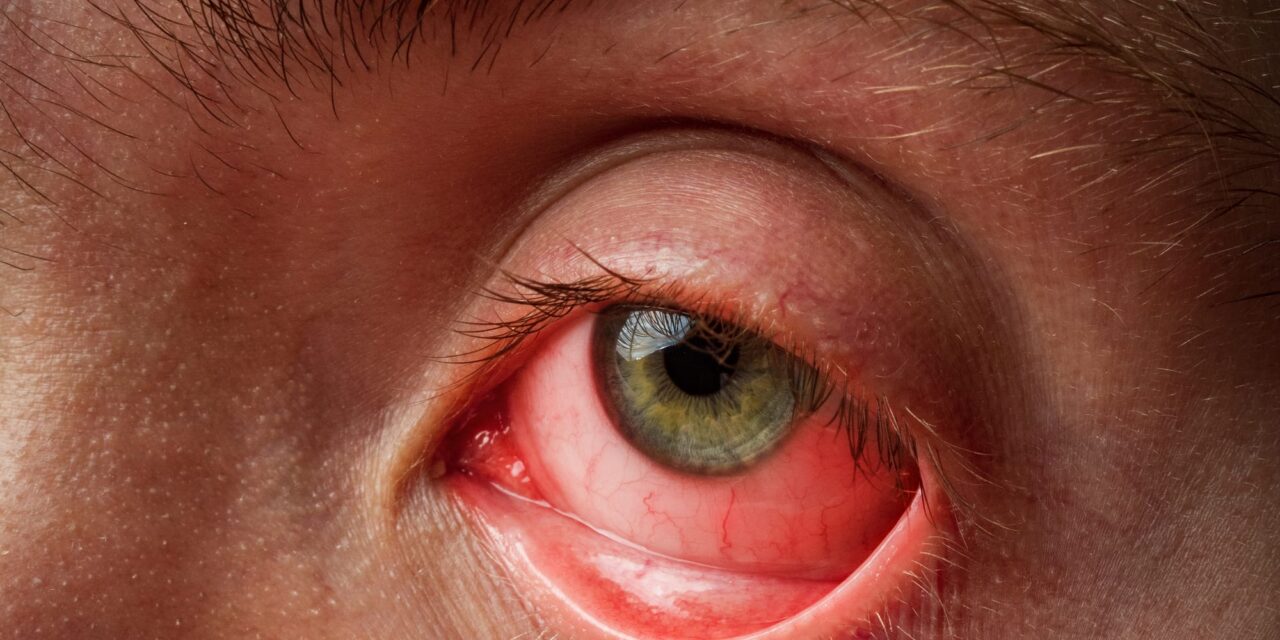
The test is being developed to differentiate between adenoviral conjunctivitis, herpetic keratitis, and allergic conjunctivitis at the point of care.
DiagnosTear Technologies announced a collaboration with AlyaTec, a contract research organization specializing in allergy and environmental diseases, to conduct a clinical study of its TeaRx Red Eye diagnostic test.
The study is designed to advance the global commercialization of TeaRx Red Eye and strengthen its FDA submission pathway. TeaRx Red Eye is being developed as a rapid, multi-biomarker diagnostic test designed to differentiate between adenoviral conjunctivitis, herpetic keratitis, and allergic conjunctivitis at the point of care.
The clinical study will examine documented allergy patients and collect tear samples before and after a controlled provocation of ocular allergy. Thirty patients are planned for enrollment, with approvals and site setup expected to take approximately three months, followed by two to three months of patient recruitment.
All samples will undergo independent, quantitative testing of total tear IgE, and results will be compared to DiagnosTear’s TeaRx Red Eye assay. The work is designed to finalize validation and analytical characterization of tear IgE as a biomarker for allergic conjunctivitis and to reinforce the company’s scientific case for its Red Eye diagnostic panel.
“This collaboration with AlyaTec represents a strong accelerator for our Red Eye program,” says Dr Shimon Gross, chief executive officer of DiagnosTear, in a release. “Accurate differentiation of red eye conditions has been a long-standing unmet need. Our goal is to deliver a test that gives clinicians immediate clarity, improves patient management, and reduces unnecessary treatments. This study moves us significantly closer to that vision.”
According to DiagnosTear, the three conditions that TeaRx Red Eye targets drive hundreds of millions of clinic visits annually, yet misdiagnosis remains widespread due to the absence of fast, accurate testing. The test is designed to deliver clinical answers in minutes.
Gross added that with the AlyaTec collaboration now underway, the company believes TeaRx Red Eye is positioned to become one of the most clinically impactful and commercially significant innovations in ophthalmic diagnostics.
Pending successful validation, DiagnosTear plans to advance additional clinical trials to support FDA clearance and prepare for global commercialization.
DiagnosTear Technologies develops point-of-care diagnostics for ocular diseases, creating multi-parametric tests that provide clinical insights based on tear fluid analysis. AlyaTec is a French clinical research organization known for its clinical models and translational expertise in allergy research.
ID 179572354 © Dubovdaniilyu | Dreamstime.com