Researchers develop PIPscore using plasma proteomics to identify triple-negative breast cancer patients most likely to benefit from immunotherapy treatment.
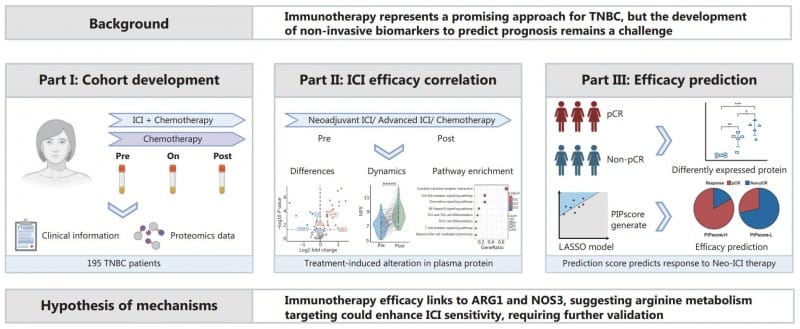
Researchers from Fudan University Shanghai Cancer Center have developed a blood-based test that predicts immunotherapy efficacy in triple-negative breast cancer (TNBC) patients with 85.8% accuracy, potentially addressing a critical gap in personalized cancer treatment.
The study, published in Cancer Biology & Medicine, analyzed plasma samples from 195 TNBC patients using high-sensitivity assays to track 92 immune-related proteins before, during, and after immunotherapy. The research team identified three key protein biomarkers—ARG1, NOS3, and CD28—and developed the PIPscore, a predictive model that could help clinical laboratories offer precision medicine tools for oncology practices.
TNBC represents an aggressive breast cancer subtype lacking targeted therapies, making immunotherapy a promising but unpredictable treatment option. Current biomarkers such as PD-L1 expression and tumor mutational burden often fail to reliably predict treatment success, while invasive tumor biopsies are impractical for frequent monitoring.
Plasma Proteomics Reveals Treatment Response Patterns
The study revealed significant shifts in plasma protein levels following immunotherapy, with immune-activating proteins like CXCL9 and IFN-γ rising in treatment responders. Patients achieving pathologic complete response demonstrated higher ARG1 and CD28 levels but lower NOS3 levels, suggesting these proteins regulate immune activation and tumor suppression.
The PIPscore combines six proteins, including ARG1, NOS3, and IL-18, to stratify patients into high- and low-response groups. High PIPscores correlated with better treatment outcomes, while low scores indicated resistance. The model demonstrated particular strength in predicting 12-month progression-free survival with 96% accuracy.
Single-cell RNA sequencing validated the plasma findings by linking protein levels to tumor microenvironment changes. Elevated NOS3 levels associated with fewer CD8+ T cells in tumors, suggesting immunosuppressive effects, while ARG1’s role in arginine metabolism may enhance T-cell function. The team confirmed findings through ELISA validation, supporting the reliability of their proteomic platform.
“This study transforms how we approach TNBC immunotherapy,” says Dr Yizhou Jiang, co-corresponding author, in a release. “By translating complex plasma proteomics into a practical score, we’ve bridged the gap between research and clinical utility. The PIPscore not only predicts response but also opens doors to targeting metabolic pathways like arginine deprivation to overcome resistance.”
The study was supported by the National Key Research and Development Project of China, the Shanghai Committee of Science and Technology, and institutional projects from the Shanghai Institute for Biomedical and Pharmaceutical Technologies.
Photo caption: Study flowchart
Photo credit: NewsWise/Cancer Biology & Medicine