This is a companion article to the CLP feature, “The New Path Forward.”
Over the past few decades, there has been a dramatic increase in the incidence of esophageal cancer (EC), a highly lethal disease known to have a 5-year survival rate of just 17%. Data from the National Cancer Institute indicate that EC has increased at a pace greater than any other type of cancer, growing by 500% since the 1970s.
According to Mike Hoerres, CEO of Cernostics Inc, Danville, Pa, such an increase in EC cases has led to a need for improved diagnostic and prognostic testing for Barrett’s esophagus (BE), a precancerous condition that is considered a precursor to EC.1
There are 3 to 4 million confirmed cases of BE in the United States. Patient management decisions are currently based on histological evaluations of esophageal biopsies. This practice is limited by observer variation and cannot predict which cases of BE will evolve into EC.
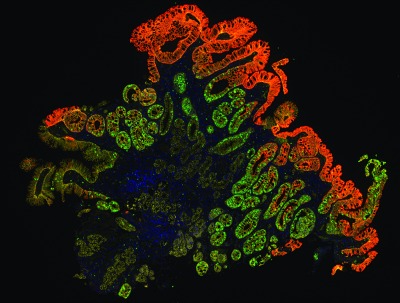
The TissueCypher technology by Cernostics quantifies multiple tissue systems biomarkers in the context of tissue morphology on a single slide, to provide accurate risk prediction for the development of esophageal cancer. This image shows a Barrett’s esophagus biopsy with epithelial (yellow) and stromal (green) infiltration of macrophages.
To address such limitations, Cernostics has developed its TissueCypher image analysis software platform to evaluate digital images from multichannel fluorescence whole-slide images. Multiple epithelial, immune, and stromal biomarkers are labeled by immunofluorescence on a tissue slide from an esophageal biopsy of BE. This information can then be converted into novel diagnostic and prognostic tests.
The Cernostics technique is undergoing field trials at a number of sites in the United States, including the Geisinger Medical Center in Danville, Pa. Jeff Prichard, MD, director of surgical pathology at Geisinger, says that by using the Cernostics technology, he gets a more accurate assessment of the esophageal biopsies of Barrett’s patients from the immunofluorescence data the platform provides.
In the future, Cernostics expects that its technique will lead to improved diagnostic and prognostic testing in patients with BE, resulting in better patient outcomes, including reductions in the incidence of EC. In addition, better risk-stratification tools will lead to increased efficiency in esophageal surveillance.
REFERENCE
1. Hoerres M, Critchley-Thorne R. Barrett’s esophagus and the need for improved diagnostic and prognostic testing. Medical Laboratory Observer. 2014;46(4):32–34.



