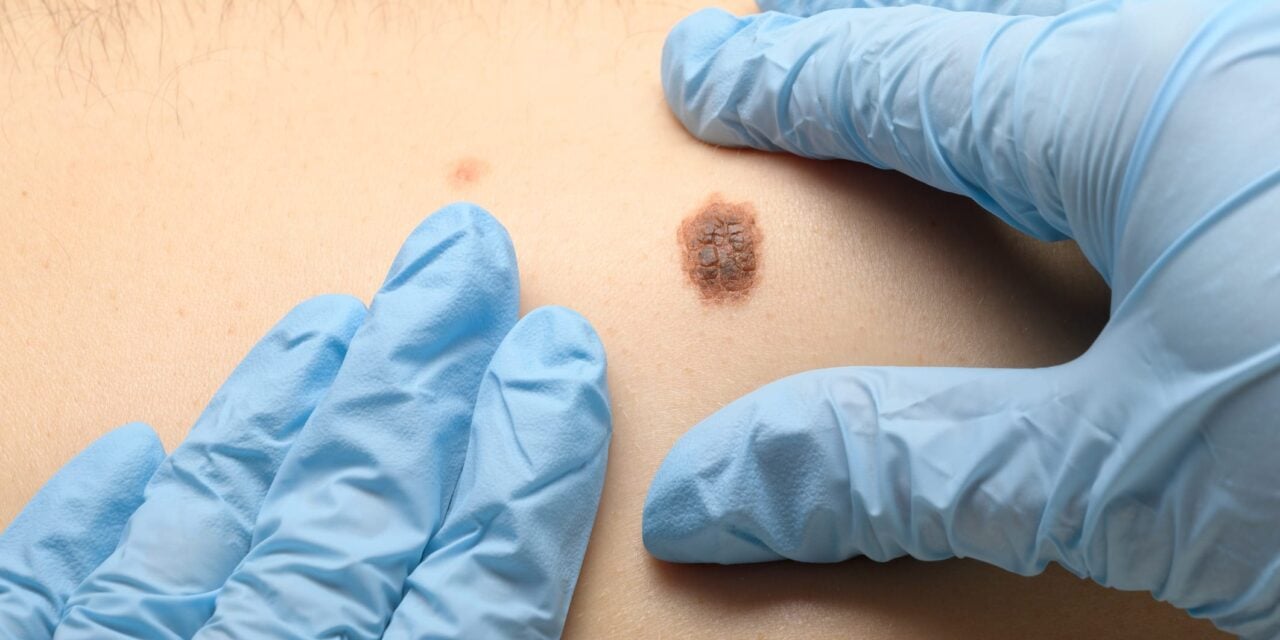
A study of nearly 1,800 patients shows a threefold difference in sentinel node metastasis rates between high-risk and low-risk groups.
Results from the MERLIN_001 trial, described as the largest prospective evaluation of a genomic test in cutaneous melanoma, have been published in JAMA Surgery, demonstrating that the Merlin CP-GEP Test can stratify T1-T3 melanomas with distinct risk levels for sentinel node metastasis.
The study enrolled 1,761 patients across nine leading US academic cancer centers and found that patients with high-risk results had a three-fold higher rate of sentinel node metastasis compared to those with low-risk results (23.8% vs 7.1%).
SkylineDx is the Rotterdam, Netherlands-based company that developed the test. The trial included patients from Mayo Clinic, University of Louisville, University of Michigan, Huntsman Cancer Institute, University of Kentucky, Emory University, Duke University, Memorial Sloan Kettering Cancer Center, and Moffitt Cancer Center.
“This study represents a major step forward in evaluating personalized melanoma care,” says Vernon Sondak, MD, MERLIN_001 principal investigator and chair of the cutaneous oncology department at Moffitt Cancer Center in Tampa, Florida, in a release. “Our results show that the test adds a level of accuracy above current clinical factors alone, even when factors like mitotic rate and histologic subtype are taken into account.”
Key Findings
- Across the study population, the Merlin CP-GEP Test stratified melanomas into two distinct risk groups: High-Risk patients had greater than a three-fold likelihood of SLN metastasis (23.8%) compared to Low-Risk patients (7.1%). Overall, 37.0% of patients were Low-Risk while 63.0% were High-Risk. Importantly, the test was successful in 97.7% of submitted samples.
- In patients aged 65 and older, where comorbidities can be more prevalent, the test identified 57.9% as High-Risk, with a positive SLNB rate of 20.3%, versus a 6.6% positive SLNB rate for Low-Risk cases.
- In head and neck melanomas, where SLNB can be technically difficult and can carry higher morbidity, the Merlin CP-GEP test identified High-Risk cases with a 26.7% SLN rate and Low-Risk cases with a 4.9% SLN (five-fold risk increase in the High-Risk vs. the Low-Risk groups).
- Across all ages and primary sites, cases with clinical Stage IB melanoma were classified as Low-Risk 49.3% of the time, with a 6.5% positive SLNB rate, compared with an 18.3% rate for High-Risk cases.
Test Combines Clinical and Genomic Data
The Merlin CP-GEP Test is a commercially available gene expression profiling test that combines clinicopathologic variables with gene expression profiling into a single integrated algorithm, according to SkylineDx. The test provides binary stratification of all patients based on high or low risk for metastasis.
The advanced CP-GEP model was developed by Mayo Clinic and SkylineDx and has been clinically validated in multiple studies globally. The test has been launched in the US and Europe.
Sondak says the test’s ability to distinguish patients’ risk of sentinel node metastasis allows patients and surgeons to make better decisions about when sentinel node biopsy should be part of managing clinically localized melanoma.
ID 176159245 © Aleksandr Malikov | Dreamstime.com