|
A Nebraska-based pioneer in control materials for hematology and other testing has developed the first human-based whole blood glucose standard—a product it expects will have a significant impact on the diabetes care industry.
Founded 36 years ago in Omaha, Neb, Streck Inc now accounts for 65% of the world market in hematology controls through distribution of its own proprietary labels, private labeling pacts, and patent licensing initiatives, according to company spokesmen.
The new blood glucose standard, according to Streck Founder and CEO Wayne Ryan, PhD, will eliminate the need for daily blood draws used during the manufacturing of instruments, test strips, and reagents. Because of the high stability level of the product’s blood and glucose, the whole-blood standard yields commutable glucose values that agree among all instruments used for testing.
The testing of blood glucose levels and the understanding of its results play a critical role in the diagnosis and care of those already with diabetes and many others who are classified as prediabetic.
Diabetes is the sixth leading cause of death in the United States. Nearly 21 million people in this country already have diabetes; as many as 54 million more Americans have elevated blood sugar levels that have not reached the diabetes threshold.
Increasingly, people with diabetes are being encouraged to take responsibility for their day-to-day care and for keeping their glucose levels from getting too low or too high.
The nonprofit American Diabetes Association (ADA) recommends tight glycemic control as a standard of care. In addition, the ADA recommends that the error in measuring glucose with such equipment as point-of-care (POC) glucometers be less than +5%.
The Streck whole-blood glucose standard mimics fresh blood for a variety of characteristics essential for consistent and accurate results. The glucose standard offers fresh-blood physical and functional characteristics—among them flow rate, viscosity, surface activity, and cellular elements. Glucose recoveries, according to Ryan, agree with whole blood across a wide variety of instrument technologies and platforms.
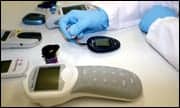 |
| The Streck Blood Glucose Standard is being evaluated as a substitute for daily fresh- blood draws used in the manufacturing of instruments, test strips, and reagents. |
The whole-blood standard replicates these precision characteristics across multiple reagent strip lots, while its glucose concentration remains stable. The Streck product, Ryan says, reduces the cost of current procedures that depend on daily fresh patient samples and the expense of manipulating samples used for producing instruments, test strips, and reagents.
Streck prepares its blood glucose standard using whole blood that is stabilized to provide up to 110 days’ stability at 6°C to 10°C. The glucose standard is developed with the same values as the fresh blood of 80, 180, and 300 mg/dL. In comparison with fresh blood, the glucose standard mimics values and any differences fall within the error of the methods. The new standard promises an end to correction codes and differing interpretations of tests using varying methods.
“At the present time, the new glucose standard is used only in a product for testing linearity,” Ryan says. “We are not sure of all the possible uses for the product. Most of the major POC glucose strip manufacturers have received samples of the blood glucose standard and are evaluating it as a substitute for the daily fresh blood draws.”
The manufacturers have reviewed product performance favorably. If they find it acceptable, this product could replace the need for daily blood draws/donors, act as a standard to QC incoming reagents, and/or conduct in-process testing associated with production of reagent test strips.
The Streck standard is produced from true blood via a number of sources; it is not a synthetic. “The research took many years in order to develop a product with red blood cells that function like fresh blood yet offer glucose stability,” Ryan says.
Patents have been filed for the product.
Glycolysis was another factor that had to be overcome as Streck researchers worked toward the development of their whole-blood glucose standard, according to Ryan. “The red blood cells in whole blood continue to metabolize plasma glucose at a rate of 5% to 7% per hour. This is also an issue for patient samples drawn in hospitals and physician’s offices where delays in sample analysis are expected,” he says. “Our ability to stabilize the red blood cells is what makes this product unique.”
That expertise in cell stabilization, according to Ryan, is a product of the company’s decades of experience in the development of hematology and flow-cytometry products.
“This background (in cell-stabilization techniques) has been very useful in the development of this new product,” Ryan says. “With all of the uses listed above, it is a substantial market. The POC glucose testing business is a multibillion-dollar market. The new Streck standard, Ryan explains, will prove beneficial for a range of procedures requiring daily fresh patient blood samples.
Boost for Proficiency Testing
Proficiency tests are performed routinely in hospitals to ensure that all of the many testing sites are performing accurate tests. “The blood glucose standard removes all questions about the sample. That allows for true evaluation of operator and instrument performance,” Ryan says.
 |
| Senior R&D Manager Brad Hunsley (standing) and Research Technician Lei Chen test the Streck Blood Glucose Standard on a variety of POC glucose meters. The Streck Blood Glucose Standard closely mimics the glucose recoveries reported with whole blood across a wide variety of POC glucose meters. |
Ryan also sees the blood glucose standard benefiting manufacturers of POC glucose strips by enabling them to manufacture to a known standard of blood glucose. Another promising market is the large proficiency testing agencies such as the College of American Pathologists and the American Proficiency Institute. “There is a definite need for improved accuracy for diabetics,” he says. “The standard should assist with this.”
Past aqueous controls for the commercial market lacked the ability to generate consistent, uniform results across varying instruments and technology platforms.
N. Gregory Miller, PhD, professor of pathology and codirector of clinical chemistry at Virginia Commonwealth University, Richmond, sees commutability as an essential attribute of any reference material used to calibrate a glucose or any other clinical method.
“Use of a standard that is not commutable with native clinical samples can actually produce erroneous results for patient testing,” Miller says. “Commutability is critical to method-calibration traceability.”
In an article he coauthored for the April 2006 issue of Clinical Chemistry,1 Miller and his colleagues discussed the evolution of the term “commutability” and the significance it plays in the operation of the modern clinical diagnostics laboratory.
“Historically,” they wrote, “the importance of commutability for harmonization and standardization of results in laboratory medicine has been poorly appreciated.”1
Miller and his colleagues say that a fundamental goal of laboratory medicine is that results for patients’ samples will be comparable independent from the medical laboratory that produced them.
“When a reference material is intended to be measured by a routine clinical method, commutability must be validated among all of the methods that will use the material, including the reference measurement procedure when appropriate,”1 the authors wrote.
They pointed out that noncommutability of a reference material can be caused by a matrix alteration or by a non-native analyte. “A matrix effect or a matrix bias can be caused by differences in sample matrix between the reference material and the native clinical samples,”1 the authors wrote.
The report states: “Routine measurement procedures of acceptable analytical specificity that have calibration traceable to the same higher-order reference material or reference measurement procedure should produce numerical values for clinical samples that are comparable irrespective of time, place, or laboratory generating the result.”1
High reagent-stability levels are important, Miller says, because in general, the more stable a reagent is, the more practical it is for use in clinical methods.
As for where diabetes testing is headed in the future, Miller says that noninvasive or minimally invasive testing will prove a more attractive approach for patients who monitor their glucose more frequently, while enabling them to maintain tighter control. “Tight control has been shown to reduce complications from diabetes,” he says.
More Appropriate Test Interpretations
John B. Buse, MD, PhD, director of the Diabetes Care Center at the University of North Carolina-Chapel Hill, also serves as president-elect of science and medicine on the ADA’s volunteer board. In his extensive research, Buse has evaluated both treatment and prevention strategies for diabetes, and the complications it holds for both adult and pediatric age groups.
Buse sees the new blood glucose standard as an important step ahead. “There is great variability across instruments, which makes for substantial confusion,” he says. “We need a standard measure of glucose.” Increasing the commutability of more stabilized glucose values, according to Buse, will lead to more appropriate interpretation of results.
Asked about testing trends and their impact on the care and treatment of today’s diabetics and millions of other patients in the future, Buse says the increasing popularity of continuous glucose sensors could make a big difference. “They are available now, but insurance doesn’t cover them. They can be life-saving.”
Nicholas Borgert is a contributing writer for CLP. For more information, contact .
Reference
- Miller WG, Myers GL, Rej R. Why commutability matters. Clin Chem. 2006;52:553-554.