Image: GCK gene
Although sequencing of our genetic material is becoming a routine diagnostic analysis, it is unfortunately far from simple to determine whether specific small differences in our DNA affect our risk of developing disease. The usefulness of DNA sequencing is therefore often limited to the few cases where it is already known if a gene variant increases the risk of disease. But new understanding of the GCK gene and its role in the development of diabetes could help change this.
Researchers at the Department of Biology, University of Copenhagen, have now contributed to solving this problem for a specific gene called GCK. The study has just been published in Genome Biology.
“The GCK gene, which codes for the enzyme glucokinase, regulates the secretion of insulin in the pancreas. GCK gene variants can therefore cause a form of hereditary diabetes,” explains Rasmus Hartmann-Petersen, Professor at the Department of Biology. “Although the connection between GCK and diabetes has been known for several years, we have, until now, only known the effect of a few percent of the possible variants of this gene.”
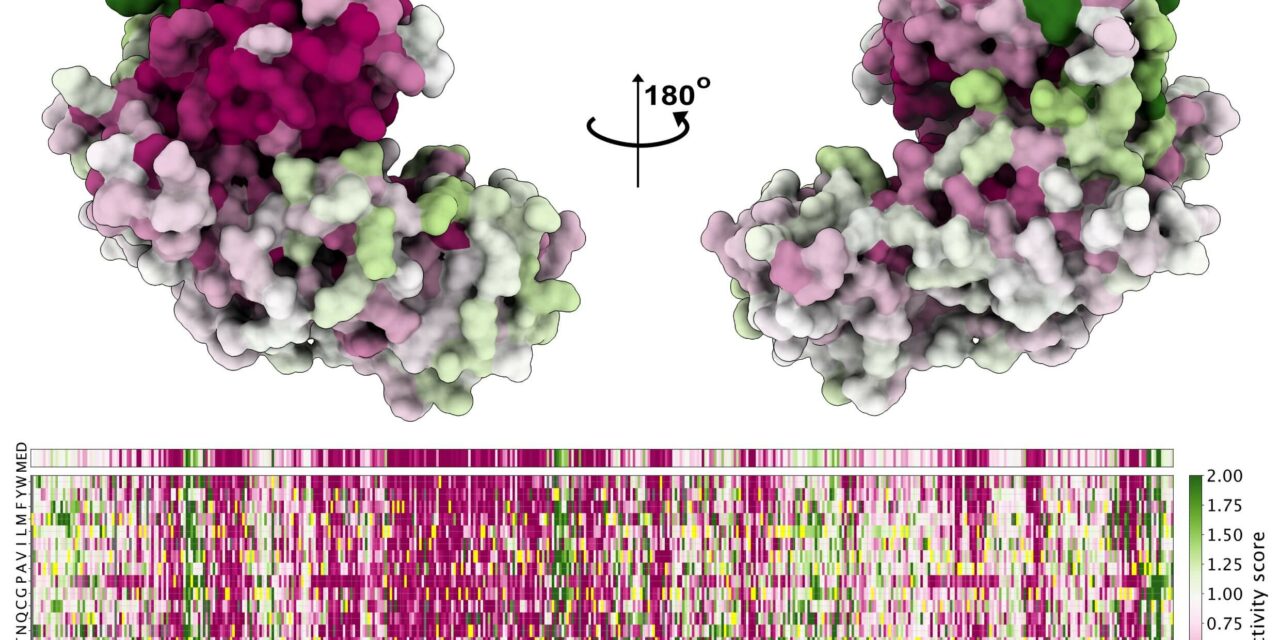
Together with colleagues at the University of Copenhagen PRISM center who are currently studying the effects of genetic variations, the researchers measured the effect of all of the possible variants of GCK.
“We used yeast cells to measure the activity of over 9,000 different GCK variants,” says PhD student Sarah Gersing, who is the first author of the article. “In this way, we were able to generate a list of the effects — both of already known variants, and of variants that patients might carry but that have not yet been discovered. This provides us with a reference for future GCK diagnostics.”
Professor Kresten Lindorff-Larsen, who heads the PRISM center, continues, “Our results are quite unique; not only have we measured the effect of several thousand variants, but for many of the variants, we can now explain what they do to the glucokinase protein. In our center, we have gathered researchers working across a range of research fields, bridging from data analysis and biophysics to cell biology and medicine, and it is now clear how this broad approach pays off in explaining how diseases arise.”
Gene variants of GCK can, among other things, cause a form of hereditary diabetes called “GCK maturity onset diabetes of the young” (GCK-MODY).
“Although GCK-MODY patients exhibit elevated blood glucose levels, this is often not associated with complications. Hence, unlike other forms of diabetes, most GCK-MODY patients might therefore not need to be treated with medication,” says Professor of Genetics Torben Hansen, MD, PhD, who is also a member of the PRISM center. “However, due to missing or inaccurate genetic data, more than half of the GCK-MODY patients are classified with having either type 1 or type 2 diabetes – and are therefore unnecessarily medicated.
“We estimate that approximately 1% of those who have recently been diagnosed with type 2 diabetes in Denmark have a variant in the GCK gene, meaning that they don’t need treatment, or need to be treated differently. Our new map of GCK variants can hopefully help give these patients a more correct diagnosis.”
Professor of Genetics Torben Hansen, MD, PhD
The next step for PRISM is to transfer these methods to other genes and diseases, a process which the researchers say is already underway, starting with genes involved in neurodegenerative diseases.
“Now, we have measured the effects of almost all variants of GCK, giving us knowledge about which variants function, and which do not. The next step is to understand why, and how the same underlying molecular mechanisms can give rise to a wide range of different diseases,” concludes Gersing.