Summary: Researchers at Karolinska Institutet and the University of Gothenburg have identified two types of metabolic-associated fatty liver disease (MASLD), offering insights for improved diagnosis, treatment, and precision medicine approaches.
Takeaways:
- New MASLD Subtypes: Researchers identified two MASLD types—liver-specific and systemic—highlighting differing clinical trajectories and risks for liver damage versus cardio-renal diseases.
- Genetic Risk Insights: Analysis of genetic data revealed 27 new variants linked to MASLD and enabled the development of risk scores for predicting disease progression and tailoring treatments.
- Advancing Precision Medicine: These findings underscore the importance of genetic research in developing personalized treatment plans and understanding the interplay between genetic and environmental factors in complex diseases.
Researchers at Karolinska Institutet and the University of Gothenburg have identified two types of metabolic-associated fatty liver disease, a liver-specific type and a systemic type that affects other organs and tissues. The discovery could lead to improved diagnosis and treatment of this growing patient group. Two studies are published back-to-back in Nature Medicine.
Impact of MASLD
Metabolic dysfunction-associated steatotic liver disease (MASLD) is characterized by an excessive accumulation of fat in the liver, which can lead to severe liver damage such as cirrhosis and liver cancer.
MASLD is caused by overweight and obesity and it is a major and growing burden globally. It is estimated that one in four adults worldwide live with MASLD, but most people are unaware because it only becomes symptomatic at an advanced stage.
Comprehensive Analyses of MASLD
Researchers at Karolinska Institutet and the University of Gothenburg in Sweden have now identified two different types of MASLD by analyzing data from over 36,000 participants from the UK Biobank and from other studies.
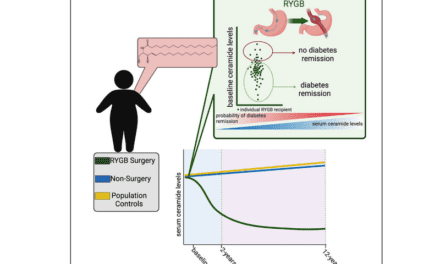
“We discovered that there are at least two types of steatotic liver disease with different clinical trajectories,” says Stefano Romeo, professor at the department of medicine, Huddinge, Karolinska Institutet, who led the research. “One is more aggressive and mainly affects the liver, while the other is entwined in the cardio-renal-metabolic syndrome.”
Predicting MASLD Progression
The researchers used genetic tests to identify 27 new genetic variants linked to MASLD. By analyzing these genes, they were able to determine two different risk scores related to the two types of MASLD. The liver-specific type is more aggressive and can lead to severe liver damage but protects against cardiovascular disease, while the systemic type is associated with a higher risk of diabetes, cardiovascular disease, heart, and kidney failure.
“This discovery is important because it helps us understand why some individuals develop more severe liver diseases while others suffer from cardio-renal diseases,” says Romeo. “This will allow us to better predict the progression of these disease and tailor the treatment to the specific needs of the patient.”
Two Parallel Studies
The back-to-back publication that professor Romeo co-led with researchers at Lille University, France, showed similar results by using another method, so-called unsupervised clustering.
“This work on clustering using simple clinical variables is of utmost importance because it allows us to differentiate at an individual level who has MASLD and will develop cardiovascular disease and who will not,” says Romeo.
The cluster prediction can be obtained by using a simple calculator.
Further Reading
Advances in Precision Medicine
The study also highlights the importance of genetic research in understanding complex diseases like MASLD and the mechanisms causing the cardio-renal-metabolic syndrome.
“This research is a step forward towards precision medicine, where treatments are tailored to suit individual needs based on genetic and clinical information,” says Romeo. “It can also raise awareness of how genetic and environmental factors affect our health and underlines the importance of continued research in this area.”
The research was mainly funded by the Swedish Cancer Society, the Swedish Research Council, ALF funds, the Swedish Heart-Lung Foundation, the Knut and Alice Wallenberg Foundation, and Novo Nordisk Foundation. Several potential conflicts of interest are listed in the article. Romeo has consulting for AstraZeneca, GSK, Celgene Corporation, Ribo-cure AB and Pfizer over the past five years and has received a research grant from AstraZeneca.