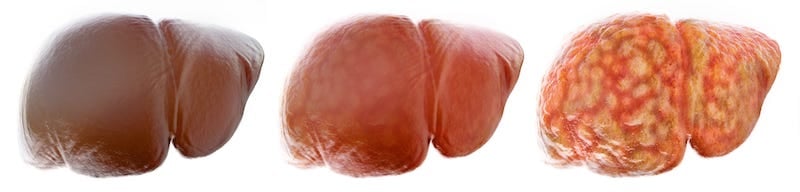
NAFLD, recently reclassified as metabolic dysfunction-associated steatotic liver disease (MASLD), is estimated to affect about 32.4% of the world’s population.
By H. Roma Levy, MS; Siming Bayer, PhD; Arati Gurung, PhD; Manuel Stich, PhD; and Jorge Rosado Sotomayor
Between 2019 and 2022, the world witnessed one of the most frightening pandemics of our time. As of mid-August 2023, the World Health Organization (WHO) confirmed almost 770 million cases of COVID-19 worldwide, resulting in just under 7 million reported deaths and leaving ~152 million survivors with significant post-COVID injury.1, 2 But while the impact of COVID has loomed large on the world stage for the last three years, nonalcoholic fatty liver disease (NAFLD) has rapidly expanded over the last two decades to become an even more significant, albeit silent, pandemic. A systematic review published in 2022 estimated NAFLD, recently reclassified as metabolic dysfunction-associated steatotic liver disease (MASLD), now affects ~32.4% of the worldwide population, that is, ~2.6 billion individuals in total, with new disease occurring at a rate of 46.9 cases/1000 person-years.3 Due to the nature of the disease, however, its growth has been largely silent and MASLD is now the most common type of chronic liver disease. Despite this, it is still poorly recognized by much of the healthcare community and political bodies the world over. 4, 5
Steatotic Liver Disease Pathophysiology
Metabolic dysfunction-associated steatotic liver disease is defined as having liver steatosis (≥5% fat per overall liver mass) and at least one of five cardiometabolic risk factors (hypertension, hypertriglyceridemia, hypercholesterolemia, above-normal BMI, glucose resistance). It is primarily driven by overweight or obesity, combined with metabolic dysfunction frequently associated with poor glycemic control (e.g., prediabetes or type 2 diabetes mellitus), although multiple factors play into its development and progression.
While prevalence is, in general, higher in industrialized nations due to ready access to an over-abundance of calorie-dense foods and an environment encouraging sedentary lifestyle, prevalence in many industrializing nations is increasing rapidly as western lifestyle is increasingly adopted.6, 7 In both developed and developing nations, MASLD is not necessarily a disease of affluence, however: it is more likely to affect socio-economically depressed populations—especially in affluent nations—due to inequities in healthcare access, poorer educational levels, and limited access to affordable healthy food options such as fruits, vegetables, and unprocessed foods in general.8
While metabolic dysfunction-associated steatotic liver disease progresses only slowly or remains stable in ~70%–75% of those affected, 25%–30% develop a more aggressive and progressive form of the disease: metabolic dysfunction-associated steatohepatitis (MASH). MASH progresses through several stages of severity characterized by several histological features, but especially the extent and pattern of liver fibrosis. The stage F0 defines those with histological evidence of inflammatory hepatocyte damage (lobular lipid infiltration, cytoplasmic ballooning) without fibrosis, while stages F1 and F2 indicate increasing degrees of mild to significant fibrosis. Severe bridging fibrosis is classified as F3, and F4 fibrosis is referred to as cirrhosis, which can be either compensated or decompensated8,9,10. Advanced fibrosis comprising stages F3 and F4 affects ~20%–30% of those with MASH (i.e., ~4%–6% of all affected).10 Advanced disease is associated with considerable morbidity and mortality and is now the second greatest and most rapidly increasing reason for liver transplantation in the U.S. and Europe.11, 12 MASLD and MASH significantly increase the risk of developing hepatocellular carcinoma, which in turn contributes to the growing rate of MASH-related liver transplant.13
Costs for care of patients with decompensated cirrhosis, hepatocellular carcinoma (HCC), or MASH-related liver transplant are six to 13 times greater than for those with either MASLD or MASH without advanced disease.14 This speaks strongly to the need for early recognition of metabolic dysfunction-associated steatotic liver disease in populations with high prevalence and therefore increased risk of the disease. For example, MASLD was noted by various studies in 50% to 74% of those with type 2 diabetes, and in 43% to 67% of those with two to five features of metabolic syndrome7, 15. Several national and international guidelines recommend progressive screening algorithms using combinations of blood biomarkers and elastography (imaging) for noninvasive identification and staging and provide guidance for whether individuals can be counseled and monitored in primary care settings or if specialist care is required.16-18
Diagnosing Patients with MASLD and MASH: Imaging-Based Technologies
Ultrasound-Based Technologies
One of the primary challenges in managing MASLD/MASH is identifying its severity without resorting to invasive procedures such as liver biopsies. Technological advancements have now granted clinicians the ability to gauge metabolic dysfunction-associated steatotic liver disease severity without invasive measures like liver biopsies. One of the state-of-the-art non-invasive imaging techniques is ultrasound, traditionally utilized for qualitative assessment of liver steatosis. Quantitative ultrasound (QUS) based methods utilize radiofrequency ultrasound signals to derive multiple quantitative ultrasound parameters such as attenuation coefficient (AC) and backscatter coefficient (BSC) that can be used to quantify liver fat content19. Most commercial ultrasound systems use only a single parameter, specifically the AC, to assess liver steatosis20.
Ultrasound derived fat fraction (UDFF) is a relatively new technique and the only commercially available technology that quantifies hepatic steatosis using multiple QUS parameters, specifically both AC and BSC21. Recent studies demonstrate the superiority of UDFF in comparison to traditional qualitative ultrasound–based methods for the detection of steatosis22. While traditional ultrasound imaging can detect fatty liver, quantitative methods such as UDFF offer a more precise measure of liver fat content, aiding clinicians in monitoring disease progression or regression.
Another advanced ultrasound-based imaging technique is the so-called point shear wave elastography (pSWE) that quantifies liver stiffness by measuring the speed of shear waves generated within the tissue using acoustic radiation force impulse (ARFI) imaging23. Shear wave speed is commonly used to evaluate liver fibrosis or scarring. The most recent advancement includes the multi-sample pSWE technology known as Auto pSWE, which allows 15 measurements in a single breath hold while maintaining diagnostic accuracy comparable to conventional pSWE24.
Magnetic Resonance-Based Technologies
While ultrasound-based measurements for liver steatosis and fibrosis are relatively inexpensive and have higher accessibility, magnet resonance imaging (MRI) based measurements provide a more comprehensive, highly reproducible, and user-independent quantification of the entire organ. MRI-derived proton density fat fraction (MRI-PDFF) quantifies the accumulated fat in liver in 3D by differentiating between the MR signals generated by fat protons and water protons in liver tissue, whereas MRI-based spectroscopy (MRS) measures the proton signals in a very small area (Voxel) as a function of their resonance frequency to calculate the fat-signal fraction. MRS suffers from relatively long acquisition times but is very sensitive to even trace amounts of liver fat and is considered the most accurate non-invasive method to quantify liver fat25. Studies have indicated that PDFF correlates well with liver biopsy results for grading hepatic steatosis26 and has the advantage to generate data for the entire organ within a single breath-hold. Meanwhile, MRI-PDFF is considered a reliable and accurate measure of hepatic steatosis and is therefore particularly useful in diagnosing and monitoring metabolic dysfunction-associated steatotic liver disease and metabolic dysfunction-associated steatohepatitis status27. Moreover, current studies22,28 have unveiled that UDFF and MRI-PDFF show strong agreement.
Magnet resonance elastography (MRE) is another emerging non-invasive imaging technology that measures liver stiffness to assess liver fibrosis. It employs MRI to visualize the mechanical waves propagating through the liver tissue, yielding a detailed elastogram which provides information about the mechanical properties (relative shear stiffness) for several representative slices through the whole liver in a breath-hold29. A recent clinical validation study demonstrates the superiority of MRE compared to transient elastography (TE) to perform liver fibrosis staging, posing a novel quantification tool for liver stiffness that is less operator-dependent30. With excellent intra-observer and inter-observer repeatability, MRE is considered the most accurate non-invasive imaging biomarker for fibrosis with equivalent statistical accuracy to biopsy31,32.
Early Detection and Surveillance of HCC
The highest risk patients—those with advanced (F3 or F4) fibrosis—are at the greatest risk of developing severe outcomes, e.g.., progressing from F3 fibrosis to cirrhosis, or from cirrhosis to a decompensatory event (new ascites, hepatic encephalopathy, esophageal varices or variceal hemorrhage), the need for liver transplant for any reason (including HCC), or death. Currently, there is only one testing method that has been granted FDA marketing authorization for prognostic risk assessment: the Enhanced Liver Fibrosis (ELF) Test. This test evaluates three serum biomarkers directly involved in liver fibrogenesis through their functionality in extracellular matrix deposition (hyaluronic acid [HA], n-terminal peptide of procollagen type 3 [PIIINP]) and fibrolysis (tissue inhibitor of metalloproteinase 1 [TIMP-1]). Combining serum levels using a proprietary algorithm generates a unitless score. A score ≥11.3 indicates higher risk of progression from F3 to F4, or from F4 to a severe outcome within one to four years of baseline.10, 33 For example, in an analysis of combined data from trials of simtuzumab and selonsertib for the treatment of MASH in patients with F3 and F4 fibrosis, Younossi et al. noted that 1.2% of patients with F3 (bridging) fibrosis experienced a liver-related clinical event within 16 months of baseline, while 7.3% of patients with cirrhosis experience decompensation or some type of other liver-related event.34
These data suggest that using the ELF Test can help clinicians determine which of their patients are at the greatest risk of progressing to cirrhosis. As cirrhosis is a well-established risk factor for hepatocellular carcinoma, use of the ELF Test to identify patients most at risk of progressing to cirrhosis offers hope for early intervention strategies and surveillance.35 To date, an effective surveillance program often involves semi-annual or annual screenings using abdominal ultrasound to detect early signs of HCC. Another potential candidate for HCC early detection and surveillance is MRI, as it provides superior soft tissue contrast resolution, facilitating the distinction between hepatocellular carcinoma (HCC) and adjacent hepatic parenchyma. Additionally, its multi-parametric imaging capabilities enhance tumor phenotyping, while the lack of ionizing radiation ensures patient safety during longitudinal monitoring, and dynamic contrast-enhanced sequences aid in evaluating lesion vascularity, which is critical for HCC diagnosis.
Consequently, the utilization of an advanced blood biomarker such as ELF to predict the risk of progression to cirrhosis, combined with other non-invasive imaging techniques for the early detection of HCC, have the potential to significantly improve outcome and prognosis by expanding the therapeutic options available, including curative treatments such as ablation, resection, or liver transplantation. Furthermore, continuous surveillance and monitoring of HCC are crucial to track disease progression, assess therapeutic efficacy, and promptly identify potential recurrences or metastases, ensuring timely intervention and optimized patient outcomes.
Altering Disease Trajectory
The clinical and economic burdens of metabolic dysfunction-associated steatotic liver disease’s end-stage complications are formidable. By emphasizing early detection through non-invasive modalities, clinicians can institute interventions sooner, potentially altering the disease trajectory. Furthermore, early detection of HCC and its subsequent treatment not only reduce costs, but more importantly, extend life and significantly improve its quality.
While the silent pandemic of MASLD continues its relentless spread, the medical community’s efforts are focused on advancing non-invasive diagnostic tools and refining surveillance protocols. With these in place, we are better positioned to confront the challenges posed by MASLD and its more aggressive counterpart, MASH.
AACE Sets Guidelines for Diagnosis and Treatment of NAFLD
The American Association of Clinical Endocrinology (AACE) conducted a comprehensive review of literature published between January 2010 and November 2021. The guideline creation was sponsored by AACE and co-sponsored by AASLD. NAFLD is a prevalent chronic progressive disease that should be identified in primary care settings. The primary care provider’s role is critical in determining risk factors, diagnosis, and risk stratification of NAFLD. Early diagnosis and stratification can lead to the prevention of advanced fibrosis. AACE guidelines emphasize the diagnosis and stratification practices for primary care providers. If people are considered low-risk, they can continue to be managed in the primary care setting. It recommends referral to hepatology when high-risk fibrosis is suspected based on high FIB-4, TE, or ELF test. These guidelines provide simple and straightforward recommendations. Management guidelines emphasize the importance of weight loss and prevention of the progression of cardiometabolic disease. In this era of T2DM and obesity prevalence, it is extremely important to identify associated comorbidities and act on them as soon as possible.
The blood tests that are advised by the guidelines, such the FIB-4 and ELF tests, are excellent indicators of how the condition may progress. They can be easily obtained, understood, and used to help the healthcare team manage the patient. Healthcare workers, including laboratory professionals, must be aware of the recommendations to combat NAFLD and enable testing while improving patient care.
NAFLD is an area of medicine that is rapidly evolving. The goal is to prevent disease progression, which is possible with early diagnosis. New medications and treatment are under evaluation for NAFLD, and guidelines will need continuous revision to incorporate the latest advances in diagnosis and treatment.
—Yessica Sachdeva, MD
About the Authors

H. Roma Levy, MS, isa medical writer and educator for Siemens Healthineers, writing; co-authoring; or supporting multiple articles, abstracts, posters, and clinical educational presentations across many disease states. She has recently left her position at Siemens Healthineers as clinical expert for Liver to pursue other opportunities.

Siming Bayer, PhD, currently heads a research group at the Pattern Recognition Lab at Friedrich-Alexander-University of Erlangen-Nürnberg (FAU), emphasizing application of AI in various fields, such as for medical image processing. She is also as a strategic collaboration manager at Siemens Healthineers, where she coordinates liver-related research activities, working closely with clinical researchers as well as industry partners.

Arati Gurung received her PhD in Bioengineering from University of Colorado Denver with emphasis on ultrasound diagnostics. She currently leads global research collaborations for Siemens Ultrasound.

Manuel Stich, Dr. rer. nat., PhD isa member of the Product Management Team, channeling his expertise towards the advancement of solutions in the domains of liver disease and liver cancer. Beyond his role at Siemens, Manuel is deeply engaged in academia. He holds a position as a dedicated university lecturer, where he imparts his extensive knowledge and experiences to inspire and nurture the next generation of medical innovators.

At Siemens Healthineers Ultrasound Jorge Rosado Sotomayor is responsible for the global commercialization strategy of Elastography and UDFF (ultrasound derived fat fraction) to establish UDFF as the leading technology for liver fat quantification through clinical efficacy with collaborations, publications, education and market segmentation tactics, and key opinion leaders (KOLs) development strategies and campaigns, and also responsible for clinical marketing initiatives for general imaging ultrasound products.

Yessica Sachdeva, MD, is an internal medicine specialist with more than 13 years of health care experience. Her primary areas of expertise and focus lie in metabolic diseases, with a particular emphasis on non-alcoholic fatty liver disease (NAFLD) as well as obesity and diabetes.
TEXT UPDATED 12/11/2023
REFERENCES
- WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. Accessed August 25, 2023.
- Bull-Otterson L, et al. MMWR. 2022;71(21):713-7.
- Riazi K, et al. Lancet Gastro Hep. 2022;7(9):851-61.
- Wessels DH, et al. World J Hepatol. 2021;13(2):233-41.
- Lazarus J, et al. J Hepatol. 2022;74(4):771-80.
- Younossi, Z. et al. Nat Rev Gastro Hepat. 2018;15:11–20
- Estes C, et al. Hepatology. 2018;67:123-33.
- Talens M, et al. Clin Med. 2021;10(21):DOI 10.3390/jcm10215019
- Chowdhury AB, et al. Clin Exp Med. 2023;23(2):273-85.
- Sanyal AJ, et al. Hepatol. 2019;70(6):1913-27.
- Younossi ZM, et al. Clin Gastro Hepatol. 2021;19(3):580-9.
- Haldar D, et al. J Hepatol. 2019;71(2):313-22.
- Schulz M, et al. Hep Med. 2020;12;125-38.
- Wong RJ, et al. J Clin Gastroenterol. 2020;55(10): 891-902.
- Godoy-Matos AF, et al. Diabetol Metab Syndr 2020;12:60.
- Berzigotti A, et al. J Hepatol. 2021;75(3):659-89.
- Cusi K, et al. Endocrine Practice. 2022;28(5):528-62.
- Rinella ME, et al. Hepatology. 2023;77(5):1797-35.
- Ferraioli G, et al.World J Gastroenterol. 2019;25(40):6053-62.
- Ferraioli G, et al. Radiology.2022;302(3):495-506.
- Yassin L, et al. J Ultrasound Med. 2020;39(12):2427-38.
- De Robertis R, et al. Radiol Med. 2023;128:1174-80.
- Ferraioli G, et al. Ultrasound Med Biol. 2015;41(5):1161-79.
- Dillman JR, et al. J Ultrasound Med. 2023 Aug 24. doi: 10.1002/jum.16309. Epub ahead of print.
- Reeder SB, et al. Magn Reason Imaging Clin N Am. 2010;19(3):337-57.
- Permutt Z, et al. Aliment Pharmacol Ther. 2012;36(1):22-9.
- Caussy C, et al. Hepatology. 2018;68(2):763-72.
- Dillman JR, Et al. AJR Am J Roentgenol. 2022;219(5):784-91.
- Venkatesh SK, et al. J Magn Reson Imaging. 2013;37(3):544-55.
- Bi, Jet al. Annals Pall Med. 2021;10(8), 8692-870032.
- Morisaka H, et al. J Magn Reson Imaging. 2018;47(5):1268-75.
- Imajo K, et al. Clin Gastroenterol Hepatol. 2022r;20(4):908-917.e11.
- Are VS, et al. Clin Gastro Hepatol. 2021;19:1292-3 e3.
- Younossi ZM, et al. Gastroenterology 2021;160:1608-19 e13
- Shah PA, et al. Hepatology. 2023;77(1):323-338.