By Erik Martin, PhD, and Rebecca Critchley-Thorne, PhD
Summary: AI-powered spatialomics tools are helping clinicians to more accurately risk-stratify patients with Barrett’s esophagus (BE), identifying those at high risk of progressing to esophageal cancer and enabling earlier and more effective treatment.
Takeaways:
- Traditional histopathological approaches to risk-stratifying patients with BE often fail to identify high-risk patients due to the manual interpretation of biopsies, which lacks sensitivity and consistency.
- TissueCypher uses AI and spatialomics to objectively analyze biomarkers and tissue structure in standard biopsies taken during upper endoscopy, delivering personalized risk scores to patients with BE with greater predictive accuracy than traditional histopathology.
- Clinical studies show that TissueCypher significantly outperforms standard pathology in identifying patients who are at high risk of progression, supporting more precise and timely treatment decisions.
Barrett’s esophagus (BE) is a physiological response to gastroesophageal reflux disease (GERD), in which stomach contents rise into the esophagus and irritate its lining.1,2 As a result of chronic acid reflux, the lining of the esophagus may respond by changing from normal, squamous epithelial cells to acid-resistant, columnar epithelial cells, similar to those found in the small intestine.2,3 While the columnar epithelial cells may better tolerate acid reflux, this metaplastic tissue is at a higher risk of developing into esophageal adenocarcinoma (EAC), which is one of the most dangerous types of cancer.4,5
The vast majority of patients diagnosed with Barrett’s esophagus will not progress to cancer. Additionally, 89% of patients with BE receive a pathology diagnosis of non-dysplastic (ND) BE,6–8 which is considered low risk and means they have less than a 1% population-based risk of progressing to EAC within the next year.6–8 Unfortunately, among the millions of patients with NDBE, a subset of them have a significantly higher individual risk of progression. This makes identifying a patient’s personalized risk of developing cancer a critical step toward improving care and reducing the incidence and mortality of EAC.
Preventing progression is critical since EAC is one of the deadliest cancers, with a five-year survival rate of approximately 20%, which declines further with late diagnosis.5 When high-risk BE is detected early, procedures, such as endoscopic eradication therapy (EET), are highly effective in preventing its progression to cancer9,10 —underscoring the clinical need for more precise and personalized risk stratification tools to ensure timely intervention for those patients who are at highest risk while avoiding unnecessary treatment for others.
Challenges in Barrett’s Esophagus Screening and Diagnosis
Barrett’s esophagus screening and diagnosis involves an esophagogastroduodenoscopy (EGD) with biopsies,11-13 which typically entails sedation, a full-day commitment from patients, and potential out-of-pocket costs. Those hurdles can deter patients from proceeding with screening.
If BE is diagnosed, societal guidelines generally do not recommend offering EET to patients with NDBE because the population-based risk of progression is very low and there are some limited complications associated with EET that should be considered.11–13
The traditional standard of care focuses on the early detection of dysplastic changes in the BE tissue through routine surveillance EGD with biopsies. If pathology can confirm that a patient has Barrett’s esophagus with low-grade dysplasia (LGD) or high-grade dysplasia (HGD), then EET can be offered.11,14 If pathology returns an “indefinite for dysplasia” (IND) result, then the patient is prescribed intensified anti-reflux medication and is scheduled for another EGD within six months.11
Unfortunately, the diagnosis of dysplasia can be limited by variability in EGD quality among endoscopists14,15 and interobserver differences among pathologists.11,16 Moreover, histopathology can only detect abnormalities that are currently present—not those that may develop in the months and years ahead.
Precision Testing is Making an Impact
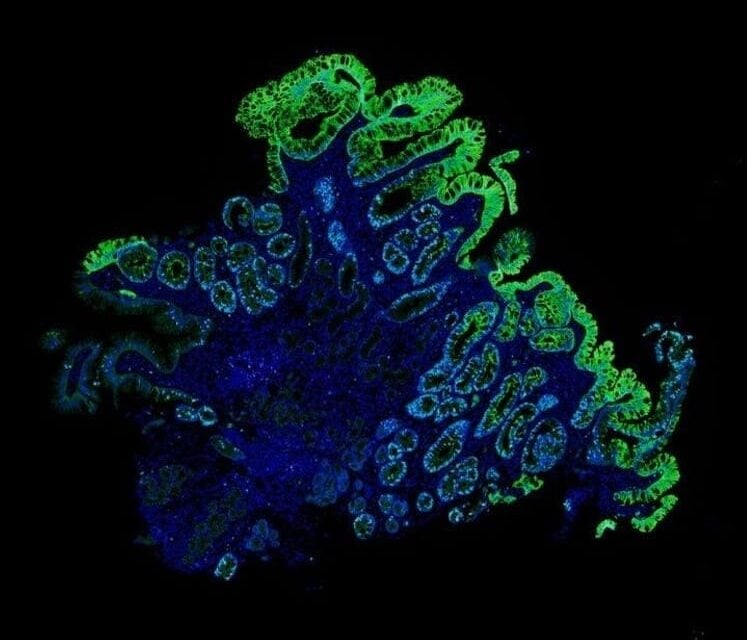
Precision medicine tools are widely utilized in the clinic to provide more accurate insight regarding patients’ individual risk of having a poor/good health outcome. One of the tools available for assessing risk in patients with Barrett’s esophagus is Castle Biosciences’ TissueCypher, which uses artificial intelligence (AI)-driven spatialomics to analyze a set of key features in tissue from standard pinch biopsies.17 While predicting the progression of BE is challenging using traditional histopathological assessments, TissueCypher offers an evidence-supported risk assessment that can help to align surveillance and treatment to each individual patient’s risk.18–25
An AI-based spatialomics approach offers numerous advantages over the traditional care approaches, including a more objective sample analysis and reducing reliance on variable and subjective human interpretation. Furthermore, digital image analysis can detect and quantify cellular features that cannot be visually interpreted by traditional histopathological assessment.
Tools such as TissueCypher may provide an effective option for identifying high-risk patients with Barrett’s esophagus who might otherwise be missed using traditional clinical approaches. This was demonstrated in a large real-world study involving more than 8,000 individuals with BE, in which researchers assessed TissueCypher’s ability to stratify patients by their risk of progression to EAC.26 TissueCypher identified patients with NDBE who scored intermediate- or high-risk and had a predicted 5-year progression risk of 8.1% and 15.3%, respectively, which are similar to and higher than published progression rates in patients with BE with confirmed LGD.26 Since LGD typically warrants therapeutic intervention to prevent EAC, this finding suggests that a significant subset of patients with NDBE may have risk levels that would justify similar clinical action, yet they would likely be missed by conventional histopathological assessment alone.
This improvement in risk stratification was observed independent of clinical factors such as age, sex, and segment length (e.g., how far the intestine-like tissue extends up the esophagus), providing evidence that TissueCypher can predict increased risk for individuals that may not be captured through traditional diagnostic approaches. The study also showed that most patients with NDBE were low-risk, and further, that low-risk subgroups also existed within patients whose Barrett’s esophagus was graded IND or LGD using only histopathological risk assessment.26
Additional findings further support the potential for improved risk stratification beyond what is possible with traditional pathology alone. For example, a pooled analysis of five published studies found that TissueCypher identified more than twice as many patients who went on to progress to HGD or EAC than histopathological assessment.23 The analysis also showed that TissueCypher’s high-risk classification was a stronger predictor of progression than clinicopathologic variables.23
Unlike traditional risk assessment using clinical and pathological factors, TissueCypher provides clinically actionable insights for individual patients. For high-risk patients who might otherwise be missed—particularly those with NDBE—this can make a crucial difference, enabling early risk assessment and timely intervention while the disease remains highly treatable.
Featured Image: One of the tools available for assessing risk in patients with Barrett’s esophagus is Castle Biosciences’ TissueCypher, which uses artificial intelligence (AI)-driven spatialomics to analyze a set of key features in tissue from standard pinch biopsies. Image: Castle Biosciences
About the Authors

Erik Martin, PhD, serves as director of Spatialomics & Gastrointestinal (GI) Research & Development (R&D) at Castle Biosciences. Martin joined Castle Biosciences in 2024 after serving as a lead scientist at Booz Allen Hamilton. There, he consulted as a subject matter expert in the biotech industry. His postdoctoral training was performed at the National Institute on Aging, a division of the U.S. National Institutes of Health, where his fluorescent microscopy and quantitative image analysis studies revealed novel insights into how the NF-kappaB pathway processes information in single cells. Martin earned his PhD in molecular medicine at the University of Maryland School of Medicine, and BS degrees in biology and biochemistry at the University of Southern Maine. He is an inventor on several patents and is the author of multiple scientific publications.

Rebecca Critchley-Thorne, PhD, serves as vice president, Research & Development at Castle Biosciences. Critchley-Thorne joined Castle in December 2021 as vice president, R&D, Spatialomics and GI, as part of the acquisition of Cernostics, Inc., where she was co-founder and chief scientific officer. She led the development of the TissueCypher computational pathology platform as well as the TissueCypher Barrett’s Esophagus test and the clinical studies supporting its use. She now oversees R&D for Castle’s commercially available tests and pipeline activities. She completed training as a postdoctoral fellow at Stanford University, where she focused on highly multiplexed analysis of biomarkers to understand mechanisms of immune dysfunction in various cancer types. She completed doctoral work in cancer immunotherapy at Imperial College and Cancer Research UK in London, and earned a BS (Hons) degree in pharmacology from the University of Sheffield, UK. Critchley-Thorne is the author of many medical and scientific publications, a principal investigator on NIH-funded research studies, and an inventor on several of Castle Biosciences’ patents.
References
- Curtius K, Rubenstein JH, Chak A, Inadomi JM. Computational modelling suggests that Barrett’s oesophagus may be the precursor of all oesophageal adenocarcinomas. Gut. 2021;70(8):1435-1440. doi:10.1136/gutjnl-2020-321598
- Naini BV, Souza RF, Odze RD. Barrett’s Esophagus: A Comprehensive and Contemporary Review for Pathologists. Am J Surg Pathol. 2016;40(5):e45-66. doi:10.1097/PAS.0000000000000598
- Maslyonkina KS, Konyukova AK, Alexeeva DY, Sinelnikov MY, Mikhaleva LM. Barrett’s esophagus: The pathomorphological and molecular genetic keystones of neoplastic progression. Cancer Med. 2022;11(2):447-478. doi:10.1002/cam4.4447
- Spechler SJ, Souza RF. Barrett’s Esophagus. N Engl J Med. 2014;371(9):836-845. doi:10.1056/NEJMra1314704
- SEER*Explorer Application. Accessed January 14, 2025. https://seer.cancer.gov/statistics-network/explorer/application.html?site=600&data_type=4&graph_type=5&compareBy=age_range&chk_age_range_1=1&series=9&sex=1&race=1&stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_show_apc=on&advopt_display=2#resultsRegion0
- Sampliner RE. Management of nondysplastic barrett esophagus with ablation therapy. Gastroenterol Hepatol (N Y). 2011;7(7):461-464.
- Merative. Merative MarketScan Commercial Claims and Encounters and Medicare Supplemental Databases. Accessed February 6, 2023. https://www.merative.com/healthcare-analytics
- Wani S, Falk G, Hall M, et al. Patients with Nondysplastic Barrett’s Esophagus Have Low risks for Developing Dysplasia or Esophageal Adenocarcinoma. Clin Gastroenterol Hepatol. 2011;9(3):220-227; quiz e26. doi:10.1016/j.cgh.2010.11.008
- Schlottmann F, Patti MG, Shaheen NJ. Endoscopic Treatment of High-Grade Dysplasia and Early Esophageal Cancer. World J Surg. 2017;41(7):1705-1711. doi:10.1007/s00268-017-3977-8
- Standards of Practice Committee – ASGE, Wani S, Qumseya B, et al. Endoscopic eradication therapy for patients with Barrett’s esophagus-associated dysplasia and intramucosal cancer. Gastrointest Endosc. 2018;87(4):907-931.e9. doi:10.1016/j.gie.2017.10.011
- Shaheen NJ, Falk GW, Iyer PG, et al. Diagnosis and Management of Barrett’s Esophagus: An Updated ACG Guideline. Am J Gastroenterol. 2022;117(4):559-587. doi:10.14309/ajg.0000000000001680
- American Gastroenterological Association, Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140(3):1084-1091. doi:10.1053/j.gastro.2011.01.030
- ASGE STANDARDS OF PRACTICE COMMITTEE, Qumseya B, Sultan S, et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest Endosc. 2019;90(3):335-359.e2. doi:10.1016/j.gie.2019.05.012
- Abela JE, Going JJ, Mackenzie JF, McKernan M, O’Mahoney S, Stuart RC. Systematic Four-Quadrant Biopsy Detects Barrett’s Dysplasia in More Patients Than Nonsystematic Biopsy. Official journal of the American College of Gastroenterology | ACG. 2008;103(4):850.
- Antony A, Pohanka C, Keogh S, et al. Adherence to quality indicators in endoscopic surveillance of Barrett’s esophagus and correlation to dysplasia detection rates. Clin Res Hepatol Gastroenterol. 2018;42(6):591-596. doi:10.1016/j.clinre.2018.06.004
- Vennalaganti P, Kanakadandi V, Goldblum JR, et al. Discordance Among Pathologists in the United States and Europe in Diagnosis of Low-Grade Dysplasia for Patients With Barrett’s Esophagus. Gastroenterol. 2017;152(3):564-570.e4. doi:10.1053/j.gastro.2016.10.041
- Prichard JW, Davison JM, Campbell BB, et al. TissueCypher(TM): A Systems Biology Approach to Anatomic Pathology. J Pathol Inform. 2015;6:48. doi:10.4103/2153-3539.163987
- Critchley-Thorne RJ, Duits LC, Prichard JW, et al. A Tissue Systems Pathology Assay for High-Risk Barrett’s Esophagus. Cancer Epidemiol Biomarkers Prev. 2016;25(6):958-968. doi:10.1158/1055-9965.EPI-15-1164
- Critchley-Thorne RJ, Davison JM, Prichard JW, et al. A Tissue Systems Pathology Test Detects Abnormalities Associated with Prevalent High-Grade Dysplasia and Esophageal Cancer in Barrett’s Esophagus. Cancer Epidemiol Biomarkers Prev. 2017;26(2):240-248. doi:10.1158/1055-9965.EPI-16-0640
- Davison JM, Goldblum J, Grewal US, et al. Independent Blinded Validation of a Tissue Systems Pathology Test to Predict Progression in Patients With Barrett’s Esophagus. Am J Gastroenterol. 2020;115(6):843-852. doi:10.14309/ajg.0000000000000556
- Frei NF, Konte K, Bossart EA, et al. Independent Validation of a Tissue Systems Pathology Assay to Predict Future Progression in Nondysplastic Barrett’s Esophagus: A Spatial-Temporal Analysis. Clin Transl Gastroenterol. 2020;11(10):e00244. doi:10.14309/ctg.0000000000000244
- Frei NF, Khoshiwal AM, Konte K, et al. Tissue Systems Pathology Test Objectively Risk Stratifies Barrett’s Esophagus Patients With Low-Grade Dysplasia. Am J Gastroenterol. 2021;116(4):675-682. doi:10.14309/ajg.0000000000001037
- Davison JM, Goldblum JR, Duits LC, et al. A Tissue Systems Pathology Test Outperforms the Standard-of-Care Variables in Predicting Progression in Patients With Barrett’s Esophagus. Clin Transl Gastroenterol. 2023;14(11):e00631. doi:10.14309/ctg.0000000000000631
- Khoshiwal AM, Frei NF, Pouw RE, et al. The Tissue Systems Pathology Test Outperforms Pathology Review in Risk Stratifying Patients With Low-Grade Dysplasia. Gastroenterology. 2023;165(5):1168-1179.e6. doi:10.1053/j.gastro.2023.07.029
- Iyer PG, Codipilly DC, Chandar AK, et al. Prediction of Progression in Barrett’s Esophagus Using a Tissue Systems Pathology Test: A Pooled Analysis of International Multicenter Studies. Clin Gastroenterol Hepatol. Published online February 22, 2022:S1542-3565(22)00190-2. doi:10.1016/j.cgh.2022.02.033
- Villa NA, Ordonez-Castellanos M, Yodice M, et al. The Tissue Systems Pathology Test Objectively Risk-Stratifies Patients With Barrett’s Esophagus: Results From a Multicenter US Clinical Experience Study. J Clin Gastroenterol. Published online July 2, 2024. doi:10.1097/MCG.0000000000002040