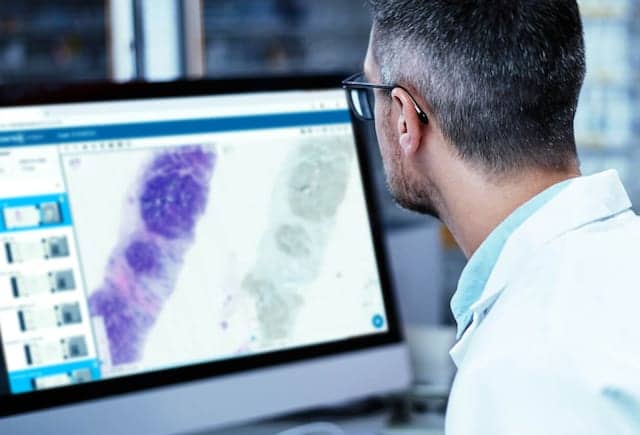
Proscia, Philadelphia, has made its Concentriq Dx digital pathology software available for use in reviewing and reporting of digital pathology slides in the United States.
In Europe, Concentriq Dx is CE-marked for use in primary diagnosis. In the United States, the software will support the need for remote pathology brought about by the covid-19 pandemic.
The company’s announcement comes as FDA has issued new guidance aimed at expanding the availability of remote digital pathology devices.
Concentriq Dx enables pathologists to securely read cases from remote locations, including home offices, using an Internet browser. The software can be rapidly deployed on existing IT infrastructure and works with any scanner and laboratory information system, offering seamless integrations with Leica, 3DHisTech, and Hamamatsu, among other leading solutions.
For more information, visit Proscia.