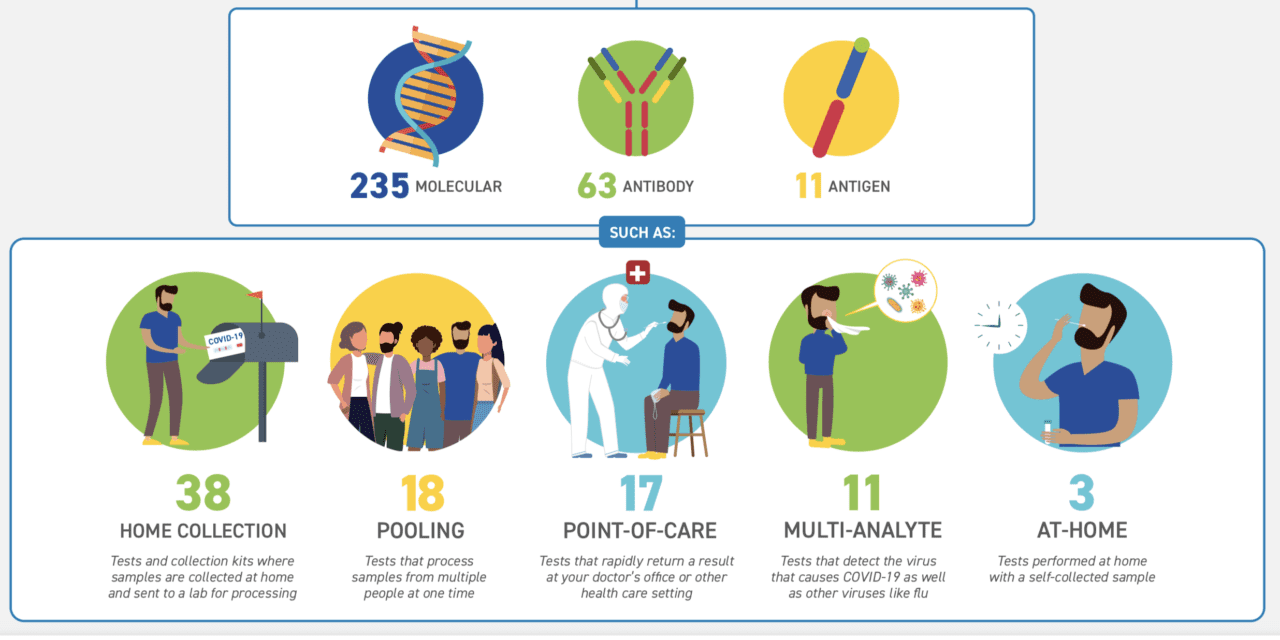
“Our Center for Devices and Radiological Health (CDRH) has made enormous contributions to the COVID-19 response. To date, the FDA has authorized 309 tests and sample collection devices. This includes a wide variety of tests, including 235 molecular tests and sample collection devices, 63 antibody tests, and 11 antigen tests, with tests that can be used in laboratories, in doctors’ offices and other point-of-care settings, and at home.” Stephen M. Hahn, M.D., Commissioner of Food and Drugs
To help the public understand their covid-19 testing options, FDA has created an infographic detailing the more than 300 covid-19 tests and collection kits authorized in 2020. The specifics include:
- 235 molecular
- 63 antibody
- 11 antigen tests
Such as:
- 38 home collection: Tests and collection kits where samples are collected at home and sent to a lab for processing
- 18 pooling: Tests that process samples from multiple people at one time
- 17 point-of-care: Tests and rapidly return a result at your doctor’s office or other health care setting
- 11 multi-analyte: Tests that detect the virus that causes COVID-19 and other viruses like flu
- 3 at-home: Tests performed at home with a self-collected sample
Current as of 12/28/2020, per the FDA.
Download the infographic pdf from FDA.