Imec, a research and innovation hub in nanoelectronics and digital technology, and miDiagnostics, a spin-off of imec in collaboration with Johns Hopkins University, announce that they have signed a non-exclusive licensing agreement for imec’s patented technology using aerosols and droplets from exhaled breath are captured for screening for viral RNA through miDiagnostics ultrafast PCR technology. The agreement enables miDiagnostics to kickstart the commercialization of a breath-based COVID-19 test.
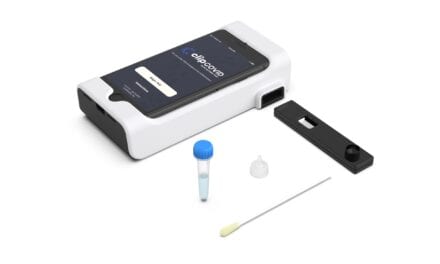
In the past year, imec developed a breath sampler based on its chip technology, and performed clinical studies with the university hospital and university of Leuven showing that its breath sampler is capable of capturing the SARS-CoV-2 virus in exhaled air, and detecting the viral RNA quickly and reliably. Also, imec developed a sampling instrument integrating the breath sampling technology and imec’s ultra-fast PCR-test, to achieve a functioning proof-of-concept model.
“I note with great pride that we have run an impressive course in the past months,” says Luc Van den hove, CEO at imec. “We have succeeded in transforming a promising concept and groundbreaking technology into a functional proof-of-concept that has passed both user tests and clinical studies. This is the first time that we have gone this far in the development of our chip technology towards commercialization. With this proof-of-concept, we can demonstrate –much closer to the market—the added value of our technology, while significantly reducing the time-to-market for our partners. The license agreement with miDiagnostics is an important milestone for imec: Our breakthrough technology will help curb the COVID-19 pandemic in the foreseeable future.”
As COVID-19 will likely not be the last virus to engulf the world, imec says it is already looking further, by investigating how its patented breath-based COVID-19 test technology can also be applied to diagnose other infectious airborne diseases, and is also looking into its application in diagnosing diseases such as cancer.
“Despite the vaccination campaigns, there’s still a great need for accessible and reliable rapid tests to curb new virus outbreaks or to avoid unnecessary quarantine. With our license to imec’s groundbreaking technology, we aim to make our ultra-fast PCR technology, which we now use for nasal swabs, also compatible with exhaled air—the perfect sample for silicon-based PCR. This first prototype will be tested at the airport in November in collaboration with Brussels Airport, Ecolog and Eurofins,” says Katleen Verleysen, CEO of miDiagnostics.
Featured Image: A demonstration sample of the proof-of-concept breath sampler, developed by imec. Photo: imec