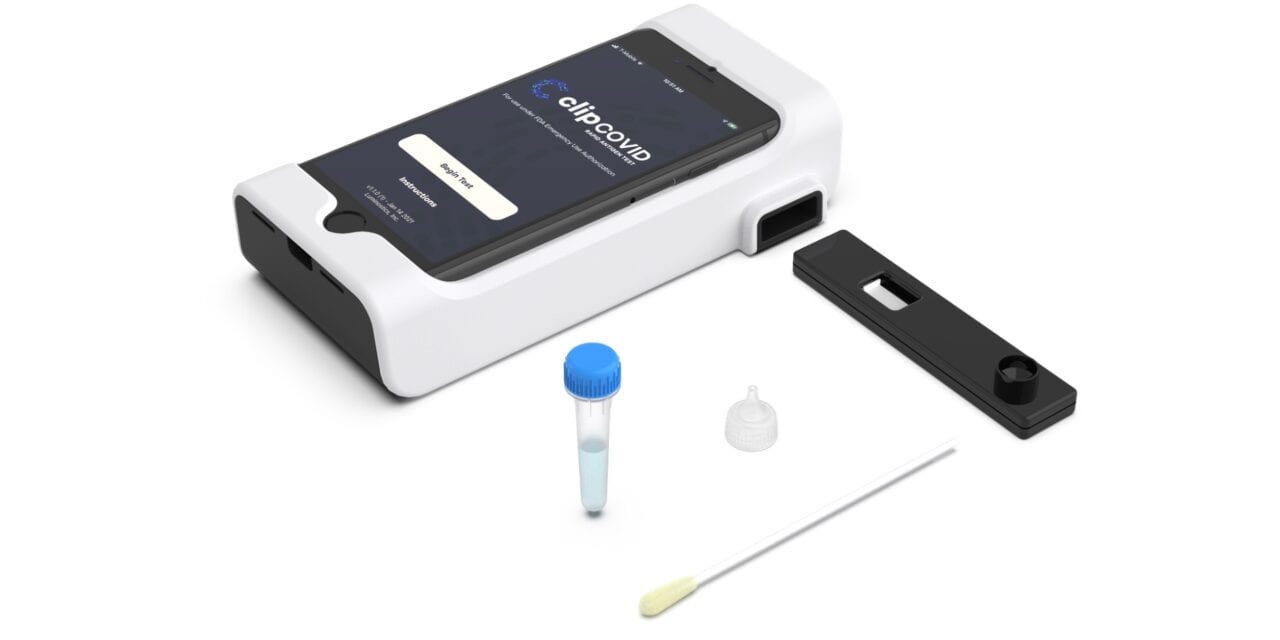
The Clip Covid Rapid Antigen Test from Luminostics, Milpitas, Calif, has received FDA emergency use authorization (EUA), and the company is scaling up production activities to help meet testing demand for covid-19.
The test is a lateral flow immunoluminescent assay that uses glow-in-the-dark nanochemistry along with a smartphone’s optics, an inexpensive adapter, and artificial intelligence to detect SARS-CoV-2 viral antigens from nasal swabs in around 30 minutes.
The Clip Covid test is among the most accurate FDA-authorized rapid antigen tests available on the market, with 100% specificity (negative percent agreement) and 96.9% sensitivity (positive percent agreement) compared to FDA-authorized, laboratory-based high-sensitivity RT-PCR in an independently run multisite prospective clinical study. Test results are objectively displayed on the smartphone’s screen. This objectivity, along with its ease of use and minimal hands-on time, enables high-throughput processing of over 30 tests per operator per hour from sample collection to result. Automated result integration to EHR and LIMS systems, as well as automated result reporting to federal, state, and local public health authorities can easily be enabled by the Clip Covid mobile app to minimize the administrative burden of organizations that run rapid covid tests.
“We are grateful to the FDA for their rapid review of our EUA application and to NIH and BARDA for their support. Our team is excited about ramping up production and helping meet the massive demand for rapid and accurate covid-19 testing. It affirms our steadfast commitment to increase our nation’s testing response so we can responsibly reopen schools, businesses, and the economy at large,” says Luminostics co-founder and CEO Bala Raja, PhD.
Luminostics expects to be manufacturing over 2,000,000 tests per month by April and more than 4,000,000 tests per month by summer. “Demand for the Clip Covid is currently far outpacing supply, but we will continue to ramp up production until this virus abates and the market tells us to stop,” Raja says. “Our immediate goals, besides ramping up production, are to expand our emergency use authorization’s labeling for non-prescription, non-laboratory use of Clip Covid.”
For more information, visit Luminostics.