A new mobile software application can help clinicians determine which patients with covid-19 are most likely to have severe cases. Created by researchers in the College of Dentistry at New York University (NYU), the app uses artificial intelligence (AI) to assess risk factors and key biomarkers from blood tests, producing a covid-19 ‘severity score.’
Current diagnostic tests for covid-19 detect viral RNA to determine whether someone does or does not have the virus, but they do not provide clues as to how sick a covid-positive patient may become.
“Identifying and monitoring those at risk for severe cases could help hospitals prioritize care and allocate resources like intensive care unit beds and ventilators. Likewise, knowing who is at low risk for complications could help reduce hospital admissions while these patients are safely managed at home,” says John T. McDevitt, PhD, a professor of biomaterials at the NYU College of Dentistry and leader of the research team. “We want doctors to have both the information they need and the infrastructure required to save lives. Covid-19 has challenged both of these key areas.”
Using data from the blood tests of 160 hospitalized covid-19 patients in Wuhan, China, the researchers identified four biomarkers that were significantly elevated in patients who died versus those who recovered: C-reactive protein (CRP), cardiac troponin I (cTnI), myoglobin (MYO), and procalcitonin (PCT). These biomarkers can signal complications that are relevant to covid-19, including acute inflammation, lower respiratory tract infection, and poor cardiovascular health.
The researchers then built a model using the biomarkers as well as age and sex, two established risk factors. Using a machine-learning algorithm, the researchers trained the model to define patterns of covid-19 disease and predict its severity. When a patient’s biomarkers and risk factors are entered into the model, it produces a numerical covid-19 severity score ranging from 0 (mild or moderate) to 100 (critical).

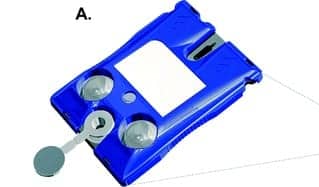
The New York University p-BNC assay system consists of a disposable cartridge (A) and a portable instrument (B). The instrument facilitates fluid movement inside the cartridge by crushing the fluid-filled blister packs on the cartridge surface. The instrument then reads the resulting optical fluorescent signal generated on bead sensors (C). Section C, from left: scanning electron microscope image of the cartridge’s bead array chip; fluorescent microphotograph of the bead sensors; an agarose bead sensor with immunofluorescent signal; illustration of a sandwich immunoassay on agarose bead fibers. Image courtesy Lab on a Chip.
The model was validated using data from 12 hospitalized covid-19 patients from Shenzhen, China, confirming that the model’s severity scores were significantly higher for patients who died versus those who were discharged.1
As New York City emerged as the epicenter of the pandemic, the researchers further validated the model using data from more than 1,000 New York City covid-19 patients. To make the tool available and convenient for clinicians, they developed a mobile app that can be used at the point of care to quickly calculate a patient’s severity score.
The app has been retrospectively evaluated in the family health centers at NYU Langone, which serve more than 102,000 patients each year as one of the nation’s largest federally qualified health center networks.
“Real-time clinical decision support tools for covid-19 can be extremely helpful, particularly in the outpatient setting, to help guide monitoring and treatment plans for those at greatest risk,” says Isaac P. Dapkins, MD, chief medical officer for the family health centers at NYU Langone.
After optimizing the clinical utility of the app, the researchers aim to roll it out nationwide in the coming weeks. It is possible that the covid-19 severity score could be integrated with electronic health records, thereby providing clinicians with actionable information at an early stage for those diagnosed with covid-19.
“We hope this tool can help identify those at high risk for adverse outcomes and reduce the health disparities present with covid-19,” says Larry K. McReynolds, executive director for the family health centers at NYU Langone.
The covid-19 severity score leverages a model McDevitt previously developed to predict outcomes for patients with cardiac disease. Cardiac health is one of several priorities of McDevitt’s lab, which creates point-of-care diagnostic systems that can be programmed to test for oral cancer, cardiac disease, and now covid-19.
The diagnostic system uses small, noninvasive samples—such as swabs of saliva or drops of blood from a fingertip—which are added to credit card-sized cartridges armed with bio-nano-chips pioneered by McDevitt. The cartridge is inserted into a portable analyzer that simultaneously tests for a range of biomarkers, with results available in less than half an hour.
Because this technology is currently used for research and informational purposes only, the covid-19 app can only be used in conjunction with existing laboratory tests and requires oversight by an authorized clinician. However, over the next few months, McDevitt’s laboratory, in partnership with SensoDx, a company spun out of his lab, plans to develop and scale the ability to test a drop of blood for covid-19 severity biomarkers—similar to how a person with diabetes tests their blood sugar—and produce a severity score on the spot.
“With covid-19, point-of-care testing, coupled with a decision-support system, could improve how clinicians triage patients—and potentially improve their outcomes, particularly for those who need more immediate and aggressive care,” says McDevitt.
Read more from NYU.
Reference
- McRae MP, Simmons GW, Christodoulides NJ, et al. Clinical decision support tool and rapid point-of-care platform for determining disease severity in patients with covid-19. Lab Chip. Epub June 3, 2020; doi: 10.1039/d0lc00373e.
Featured image:
Disposable cartridge of the New York University p-BNC assay system.