Mologic Ltd, Bedford, UK, which develops personalized diagnostics to improve the lives of patients, has received the CE mark for BVPro, a rapid point-of-care test for patients presenting with symptoms of vaginosis. The company also announced the commercial launch of the test, and that it has signed a 3-year distribution agreement with Sysmex subsidiary Hitado GmbH, a leading diagnostic supplier specializing in point-of-care products, for distribution of BVPro throughout Germany.
Bacterial vaginosis (BV) is an abnormal vaginal condition that is characterized by vaginal discharge and results from an overgrowth of atypical bacteria in the vagina. Although not critical in its own right, BV is associated with such serious medical complications as postoperative infections, greater susceptibility to such sexually transmitted infections as genital herpes and HIV, and a higher risk of preterm birth in pregnant women.
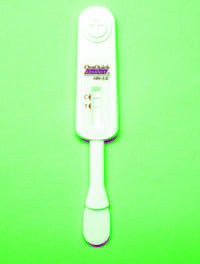
BVPro is a simple, 15-minute, point-of-care diagnostic for professional use with patients presenting with symptoms of vaginosis. It detects sialidase enzyme activity from a vaginal swab sample, which is a well-established clinical marker of bacterial vaginosis. The CE marked diagnostic has been designed in the familiar lateral-flow format, so that the visual result is easy to interpret. The test offers a rapid alternative to current methods, such as wet mount microscopic analysis and test tube-based color-change assays, which are time-consuming and often provide ambiguous results.
“BVPro is another in Mologic’s pipeline of diagnostic tests to receive CE mark approval. We are very pleased to be making such progress in bringing point-of-care tests to patients and, by doing so, enabling the earlier treatment of a range of diseases, including sepsis, urinary tract infections, and chronic obstructive pulmonary disease,” says Mark Davis, cofounder and CEO at Mologic. “Appointing Hitado to distribute our products in Germany represents a significant milestone for our company. We look forward to working with them as our first formal distributor and also others as we bring them on board in different geographies.”
For more information, visit Mologic Ltd.