 |
| HIV in the bloodstream. |
Today, the complexity of STDs requires more than a simple identification, but clinical labs rise to the occasion as technology fills in the gaps
BY: RENEE DIIULIO
Demand has always driven diagnostics: Patients want to be cured, and physicians want to cure them. But to do so, they must know what they are treating. In the beginning, it was a huge advance to be able to identify a disease, anatomical failure, or invading organism—but thanks to growing knowledge and advancing technology, simple identification is often no longer enough.
Today, the medical community is charged with not just diagnosis, treatment, and cure, but also prevention and screening. Whether a condition can or can’t be cured, avoiding it altogether is every patient’s ideal choice. Medicine does what it can, sometimes with great success. Viruses have been eradicated, or nearly so (eg, smallpox); precancerous lesions can be detected and removed before evolving into cancer; and diets can be prescribed to avoid diabetes, heart failure, and other conditions. In some instances, as with a diet, patient compliance is key. But compliance is often hard, and when it comes to sexually transmitted diseases (STDs), it comes with many challenges.
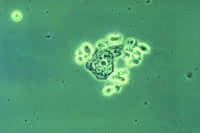 |
| This phase contrast wet mount micrograph of a vaginal discharge revealing the presence of Trichomonas vaginalis protozoa. Trichomonas vaginalis, a flagellate, is the most common pathogenic protozoan of humans in industrialized countries. This protozoan resides in the female lower genital tract and the male urethra and prostate, where it replicates by binary fission. CDC, 1986 |
Certainly, there is an entire industry devoted to minimizing the risk of STDs, but accidents happen, crimes occur, and bad luck (or bad decisions) have consequences. Research indicates that certain conditions increase the risk of acquiring a second STD (eg, trichomoniasis or gonorrhea and HIV), compounding the problem. To further complicate matters, some of these diseases can infect a host without symptoms appearing until years later, when they may manifest as cancer, infertility, or some other more difficult issue.
Even if identified early, some STDs, such as HIV, are incurable; others, such as gonorrhea, have become drug-resistant; and still others, HIV again, can mutate in vivo, requiring a change in treatment (if available). Once more, simple identification is no longer enough.
Diagnostic technologies, therefore, play a larger role than in the past in the treatment path for an STD. They may provide not only results regarding what an infection is, but also how it may be most successfully treated. In this regard, the advent of molecular technology has generally been a huge boon, and it continues to hold promise, but it has also created some gaps—which are driving technological development.
 |
“NAAT tests may be helping us do a better job at detecting gonococcal infections.”—Robert Kirkcaldy, MD, PhD, medical epidemiologist, division of STD Prevention, CDC |
Drug Susceptibility Testing for Gonorrhea
Neisseria gonorrhoeae, for instance, can now be quickly and accurately identified through nucleic acid amplification testing (NAAT), enabling physicians, in turn, to make a rapid and accurate diagnosis. “NAAT tests may be helping us do a better job at detecting gonococcal infections,” says Robert Kirkcaldy, MD, PhD, medical epidemiologist, Division of STD Prevention, Centers for Disease Control and Prevention (CDC), Atlanta.
He cites the tests’ excellent sensitivity and specificity as well as a reduction in the logistical burden of specimen handling and the ability, in some instances, to move testing out of a health care setting. But for patients who return with a treatment failure, identification is not enough.
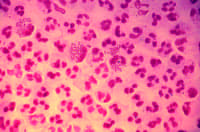 |
| This photomicrograph reveals the histopathology in an acute case of gonococcal urethritis using Gram- stain technique. |
Neisseria gonorrhoeae has been very successful at evolving antibiotic resistance. Many of today’s cases respond solely to treatment that incorporates cephalosporin, often in combination with other drugs. But a growing number are showing resistance to this course as well. The problem is more prevalent worldwide, but the CDC is monitoring the domestic situation closely in an effort to control the organisms’ spread.
This requires susceptibility testing (and reporting to the Gonococcal Isolate Surveillance Project, or GISP), which is not necessarily automatic for all physicians and/or laboratories when encountering a treatment failure. “We’d like physicians to recognize that they need to test for cure or call for drug susceptibility testing in cases where they suspect a treatment failure,” says Mark W. Pandori, PhD, HCLD(ABB), director, San Francisco Department of Public Health Laboratory.
For this reason, clinical labs should be prepared to answer the request. However, molecular methods are currently commercially unavailable to determine whether the virus is drug-resistant, and many labs—having minimized or eliminated culture methodologies while increasing automated molecular technologies—no longer have the capability in-house. This has the potential to hinder decision-making and surveillance as well as lead to empirical treatment, which can in turn lead to greater drug-resistance.
Yet culture remains the gold standard for drug susceptibility. “Some feel that may never change, since whether an organism shows sensitivity to a drug is directly related to how the drug affects growth,” says Rick Pesano, MD, PhD, medical director of infectious diseases and immunology, Quest Diagnostics, Madison, NJ.
Pesano, and other experts, do not agree, however, believing that molecular technologies hold the
 |
| The BD Max System by BD Diagnostics – Diagnostic Systems, automates cell lysis, nucleic acid extraction, PCR setup, amplification, and detection. |
promise of providing a quick answer to drug susceptibility. Some laboratories have already developed their own tests to take advantage of existing in-house systems.
“Laboratories who work with systems that have open-channel capability, such as the BD Max, can develop their own primers, probes, and tests to detect those genes and molecular sequences that are indicative of resistance,” says Chris Demiris, marketing and sales training leader, US Specialty Products, BD Diagnostics – Diagnostic Systems, Sparks, Md.
The volume for such a test does not make development commercially viable for a company such as BD, but there are others working on solutions. With the antibiotic pipeline having slowed down tremendously, the importance of surveillance has increased. Unfortunately, molecular technologies may still take some years.
In the meantime, laboratories that find culture to be a resource burden can partner with reference or public health labs that are able to perform susceptibility testing and let physicians know that the test is available, Kirkcaldy advises. “Laboratories need a contingency plan for when drug resistance is suspected,” he says, adding they should also be prepared to report drug resistance results immediately to the physician and GISP.
 |
“Most patients don’t want to wait 2 or 3 days for a result and treatment.” —Chris Demiris, marketing and sales training leader, US Specialty Products, BD Diagnostics – Diagnostic Systems |
Trichomoniasis Testing Goes Molecular
Busy physicians may not always be aware of testing capabilities, and laboratories can help with this by reminding them of existing tests or alerting them to new ones, as some advances may fall under the radar. Physicians, for instance, may not be aware that molecular testing is now available for trichomoniasis. Until recently, culture or wet mount methods have been the sole methodology for the identification of the sexually transmitted parasite.
Hologic’s Gen-Probe Diagnostics, San Diego, has introduced an FDA-approved NAAT for the detection of Trichomoniasis vaginalis using its APTIMA platform and TIGRIS automated system. The test can be run on the same samples used for Chlamydia trachomatis and Neisseria gonorrhoeae, and offers the same benefits regarding rapid turnaround and specimen logistics.
BD has plans to launch its own molecular test for the identification of trichomoniasis, ideally in 2014, to work with its system. The BD ProbeTec Trichomonas vaginalis Qx Amplified DNA Assay has already received a CE mark, and the company is compiling its FDA-clearance application for a similar test in the United States.
“Key opinion leaders have been very supportive of repeat screening for this STD—currently, the most
 |
| The Affirm VPIII Microbial Identification Test by BD Diagnostics – Diagnostic Systems, is a direct specimen DNA probe-based diagnostic test for the differential detection and identification of the causative agents for vaginitis: Candida species, Gardnerella vaginalis, and Trichomonas vaginalis. |
prevalent in the world,” Demiris says. Unfortunately, the traditional wet mount method, while highly specific, lacks sensitivity. The organisms are fragile, and locating them under the microscope requires skill and perhaps a bit of luck—you’ve got to see one to be sure. Culture is both sensitive and specific, but amplification in this media takes time versus the much quicker turnaround completed with nucleic acid amplification testing.
“Most patients don’t want to wait 2 or 3 days for a result and treatment,” Demiris says. “Tests on the BD Max are completed within 2 hours, and you can run gonorrhea and Chlamydia tests on the same sample.”
Testing Algorithms to Catch Up to Testing Technology
Advances in HIV testing have also improved turnaround, in addition to offering a number of other advantages that have the potential to positively affect patient outcome. The benefits are so great that the CDC is developing a new testing algorithm to incorporate the newest technologies; the last revision was released in 1989.
Since then, HIV diagnostics vendors have moved on to fourth-generation tests. While based on similar platforms as earlier methods, they have closed the window period to 16 days, down from 40 days in the first generation, and 22 days in the third, according to Pandori.
A shorter window period is good for both the patient and the public. “If we can start treatment closer to the time of infection, we may be able to alter the medical outcome,” Pandori says. “There’s not a lot of data yet, but early research is indicating that may be the case.”
Patients aware of their diagnoses can also halt unconscious spread of the disease. People with HIV-1, the most prevalent type of the disease, are more infectious during the early stages that typically fall within the window period. There is some thought that it is this population fueling the virus’s spread, Pandori notes.
“The ability to detect recent HIV infection may be paramount from the public health point of view toward stopping new infections and could stem transmission to some degree,” Pandori says.
Additionally, the ability to differentiate between different types of the virus has value. Although HIV-2 is rare in the United States—166 cases were reported to the CDC between 1988 and 2010—disease progression and clinical management is much different. Among HIV-1 cases, subtyping can help to determine a drug course.
 |
| The Multispot HIV-1/HIV-2 Rapid Test from Bio-Rad Laboratories, Hercules, Calif, is approved for the differentiation between the two types and is suitable for use in multitest algorithms. Such a test is expected to be part of the new testing algorithm to be released by the CDC. |
The methods for accomplishing this vary. Quest recently introduced a molecular test to produce a result for HIV tropism that predicts response to CCR5 antagonist therapy. The treatment can help to slow HIV disease progression in patients with a specific tropism type, but has no impact in those with other forms of tropism. “Genotyping is an example of how diagnostic innovation can expand clinical options for physicians while driving down costs for payors and patients,” Pesano says.
Other methods cited by the American Society for Microbiology, in addition to sequencing (either of the full-length genome or partial gene regions such as the envelope, the group-specific antigen, or the polymerase gene), include probe hybridization assays, restriction fragment length polymorphism analysis, subtype-specific PCR, combinatorial melting assays, the heteroduplex mobility assay, and serotyping (indirectly). The vast majority of these tests, however, are approved for clinical management only.
The Multispot HIV-1/HIV-2 Rapid Test from Bio-Rad Laboratories, Hercules, Calif, is approved for the differentiation between the two types and is suitable for use in multitest algorithms. Such a test is expected to be part of the new testing algorithm to be released by the CDC.
“It’s anticipated that the confirmatory test will also differentiate between the two types of HIV,” Pandori says. “Right now, the only FDA-approved option for differentiating antibodies between HIV-1 and HIV-2 that would serve as a confirmatory test is a rapid test, so you could potentially obtain a specimen, screen it, and confirm the diagnosis all within the same day.”
Discordant specimens will naturally require more time, but the new algorithm is expected to address this aspect of testing as well, directing laboratories to RNA tests rather than conventional methods. This step may provide the biggest challenge for clinical laboratories when new guidelines are released.
 |
| Scanning electron micrograph of HIV-1 budding from cultured lymphocyte. |
Many labs are already running third- and fourth-generation HIV tests, and the addition of a rapid test, while it may have a budget impact for some institutions, does not require large capital expenditure or significant technologist time. The RNA test options, however, are limited, requiring hands-on activity and offering turnarounds of 5 to 6 hours. Laboratories may want to partner with others to be able to offer this service efficiently and economically.
Ultimately, clinical laboratories have to find solutions to provide clinicians with the answers they demand. Inadequate responses can lead to greater problems down the road—for patients, through complications, and for the public, through infectious spread and the evolution of antibiotic resistance.
As the questions become more complex, laboratories must adapt, and to adapt, they often turn to vendors for the tools and technologies needed to obtain clinically useful results. Sometimes vendors are ahead of demand, such as with the rapidly evolving HIV testing options, and sometimes they are a little behind, as with drug susceptibility testing for gonorrhea, but as the gaps close and outcomes improve, diagnostic capabilities help physicians cure and care for their patients.
FAST FACTS ON STD’S
|
Renee Diiulio is a contributing writer for CLP. For more information, contact Editor Judy O’Rourke, [email protected]