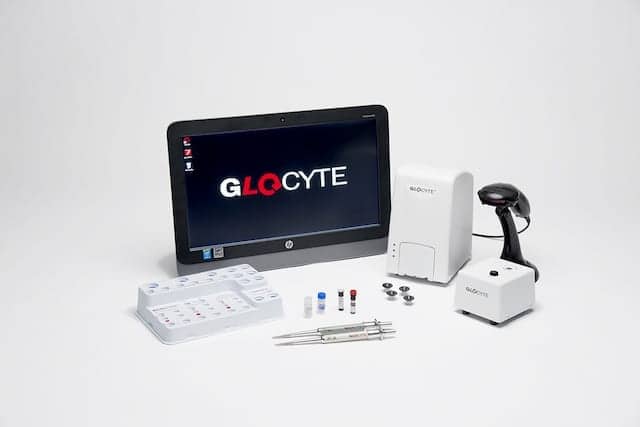
Advanced Instruments Inc, Norwood, Mass, has received FDA premarket notification (510(k)) clearance to market its GloCyte automated cell counter system and GloCyte low- and high-level controls.
The patented GloCyte system is intended to provide quantitative determination of red blood cells and total nucleated cells in cerebrospinal fluid (CSF) collected from adult and pediatric patients. CSF is collected when physicians rely on cell counts to assist in the diagnosis of infections, inflammatory processes, hemorrhage, leukemia, and malignancies that may involve the central nervous system. However, standard methods now used to detect and count cells can be challenged when the proportion of cells to be counted is extremely low.
“To date, there has not been a way to provide dependable, low cell counts,” says John Coughlin, president and CEO of Advanced Instruments. “We use a novel combination of fluorescence, microscopy with digital image analysis principles, highly specific reagents, and an intelligent counting algorithm to provide accurate and precise cell counts. We are very excited, as this marks a major achievement in giving laboratories a new way to obtain reliable and timely CSF results.”
Requiring only 30 µL of sample per test, the system uses disposable test cartridges, ensuring no sample carryover and easy disposal. The system also includes built-in quality control using Levey-Jennings charts and an audit table.
The company expects to begin GloCyte shipments in September of this year.
For more information, visit Advanced Instruments.